Efficacy and safety of modafinil film-coated tablets in children and adolescents with or without prior stimulant treatment for attention-deficit/hyperactivity disorder: pooled analysis of 3 randomized, double-blind, placebo-controlled studies
- PMID: 17245457
- PMCID: PMC1764520
Efficacy and safety of modafinil film-coated tablets in children and adolescents with or without prior stimulant treatment for attention-deficit/hyperactivity disorder: pooled analysis of 3 randomized, double-blind, placebo-controlled studies
Abstract
Objective: This report evaluated the efficacy and tolerability of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder (ADHD), diagnosed using DSM-IV-TR criteria, who did or did not receive prior treatment with stimulants for ADHD by examining pooled data from 3 randomized, double-blind, placebo-controlled studies.
Method: Three patient populations were evaluated: (1) all patients (i.e., all-patient group), (2) patients who were treated previously with stimulants (i.e., prior-stimulant group), and (3) patients who either were treated previously with ADHD medications other than stimulants or were not treated with any medications for ADHD (i.e., medication- or stimulantnaive group). Tolerability was evaluated by monitoring adverse events reported by both patients and parents. The 3 studies were conducted between November 2003 and June 2004.
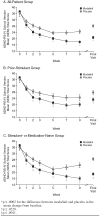
Results: Of 638 patients randomized, 633 received modafinil (N = 420) or placebo (N = 213), 303 had received prior stimulant treatment (modafinil, 194; placebo, 109), and 330 had no prior stimulant experience (modafinil, 226; placebo, 104). Modafinil improved symptoms of ADHD, as assessed by ADHD-RS-IV School Version total scores (mean change from baseline to final visit compared with placebo) in the all-patient group (-16.4 vs. -8.3) (p < .0001), the prior-stimulant group (-14.2 vs. -9.3) (p < .001), and the medication-or stimulant-naive group (-18.3 vs. -7.3) (p < .0001). Similar improvements were observed on the ADHD-RS-IV Home Version and for overall clinical condition. Insomnia, headache, and decreased appetite were the most commonly reported adverse events. Discontinuation because of adverse events was similar in the modafinil and placebo groups (5% vs. 3%).
Conclusions: This post hoc analysis extends previous findings that modafinil was well tolerated and improved the symptoms and behaviors of ADHD at school and at home as assessed by teachers, parents, and clinicians and improved patients' overall clinical condition. Improvements were shown regardless of history of stimulant use.
Figures


References
-
- Brown RT, Amler RW, and Freeman WS. et al. Treatment of attention-deficit/ hyperactivity disorder: overview of the evidence. Pediatrics. 2005 115:e749–e757. - PubMed
-
- Jensen PS, Hinshaw SP, and Swanson JM. et al. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. J Dev Behav Pediatr. 2001 22:60–73. - PubMed
-
- The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder: Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. - PubMed
-
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-ups: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics. 2004;113:754–761. - PubMed
-
- Kelsey DK, Sumner CR, and Casat CD. et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics. 2004 114:e1–e8. - PubMed
LinkOut - more resources
Full Text Sources
