Infrared spectroscopy of cationized arginine in the gas phase: direct evidence for the transition from nonzwitterionic to zwitterionic structure
- PMID: 17249666
- PMCID: PMC2675882
- DOI: 10.1021/ja066335j
Infrared spectroscopy of cationized arginine in the gas phase: direct evidence for the transition from nonzwitterionic to zwitterionic structure
Abstract
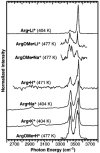
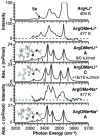
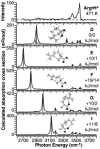
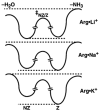
The gas-phase structures of protonated and alkali metal cationized arginine (Arg) and arginine methyl ester (ArgOMe) are investigated with infrared spectroscopy and ab initio calculations. Infrared spectra, measured in the hydrogen-stretch region, provide compelling evidence that arginine changes from its nonzwitterionic to zwitterionic form with increasing metal ion size, with the transition in structure occurring between lithium and sodium. For sodiated arginine, evidence for both forms is obtained from spectral deconvolution, although the zwitterionic form is predominant. Comparisons of the photodissociation spectra with spectra calculated for low-energy candidate structures provide additional insights into the detailed structures of these ions. Arg*Li+, ArgOMe*Li+, and ArgOMe*Na+ exist in nonzwitterionic forms in which the metal ion is tricoordinated with the amino acid, whereas Arg*Na+ and Arg*K+ predominately exist in a zwitterionic form where the protonated side chain donates one hydrogen bond to the N terminus of the amino acid and the metal ion is bicoordinated with the carboxylate group. Arg*H+ and ArgOMe*H+ have protonated side chains that form the same interaction with the N terminus as zwitterionic, alkali metal cationized arginine, yet both are unambiguously determined to be nonzwitterionic. Calculations indicate that for clusters with protonated side chains, structures with two strong hydrogen bonds are lowest in energy, in disagreement with these experimental results. This study provides new detailed structural assignments and interpretations of previously observed fragmentation patterns for these ions.
Figures









References
-
- Kassab E, Langlet J, Evleth E, Akacem Y. J Mol Struct (THEOCHEM) 2000;531:267–282.
-
- Jensen F. J Am Chem Soc. 1992;114:9533–9537.
-
- Hoyau S, Ohanessian G. Chem–Eur J. 1998;4:1561–1569.
-
- Hoyau S, Pelicier JP, Rogalewicz F, Hoppilliard Y, Ohanessian G. Eur J Mass Spectrom. 2001;7:303–311.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

