Site-directed photoproteolysis of 8-oxoguanine DNA glycosylase 1 (OGG1) by specific porphyrin-protein probe conjugates: a strategy to improve the effectiveness of photodynamic therapy for cancer
- PMID: 17251034
- PMCID: PMC1868704
- DOI: 10.1016/j.jphotobiol.2006.12.004
Site-directed photoproteolysis of 8-oxoguanine DNA glycosylase 1 (OGG1) by specific porphyrin-protein probe conjugates: a strategy to improve the effectiveness of photodynamic therapy for cancer
Abstract
The specific light-induced, non-enzymatic photolysis of mOGG1 by porphyrin-conjugated or rose bengal-conjugated streptavidin and porphyrin-conjugated or rose bengal-conjugated first specific or secondary anti-IgG antibodies is reported. The porphyrin chlorin e6 and rose bengal were conjugated to either streptavidin, rabbit anti-mOGG1 primary specific antibody fractions or goat anti-rabbit IgG secondary antibody fractions. Under our experimental conditions, visible light of wavelengths greater than 600 nm induced the non-enzymatic degradation of mOGG1 when this DNA repair enzyme either directly formed a complex with chlorin e6-conjugated anti-mOGG1 primary specific antibodies or indirectly formed complexes with either streptavidin-chlorin e6 conjugates and biotinylated first specific anti-mOGG1 antibodies or first specific anti-mOGG1 antibodies and chlorin e6-conjugated anti-rabbit IgG secondary antibodies. Similar results were obtained when rose bengal was used as photosensitizer instead of chlorin e6. The rate of the photochemical reaction of mOGG1 site-directed by all three chlorin e6 antibody complexes was not affected by the presence of the singlet oxygen scavenger sodium azide. Site-directed photoactivatable probes having the capacity to generate reactive oxygen species (ROS) while destroying the DNA repair system in malignant cells and tumors may represent a powerful strategy to boost selectivity, penetration and efficacy of current photodynamic (PDT) therapy methodologies.
Figures
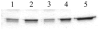


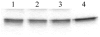


Similar articles
-
Cell cycle regulation of the murine 8-oxoguanine DNA glycosylase (mOGG1): mOGG1 associates with microtubules during interphase and mitosis.DNA Repair (Amst). 2004 Dec 2;3(12):1601-15. doi: 10.1016/j.dnarep.2004.06.011. DNA Repair (Amst). 2004. PMID: 15474421
-
Excellent antitumor effects for gastrointestinal cancers using photodynamic therapy with a novel glucose conjugated chlorin e6.Biochem Biophys Res Commun. 2018 Feb 19;496(4):1204-1209. doi: 10.1016/j.bbrc.2018.01.171. Epub 2018 Jan 31. Biochem Biophys Res Commun. 2018. PMID: 29408755
-
Immunofluorescent localization of the murine 8-oxoguanine DNA glycosylase (mOGG1) in cells growing under normal and nutrient deprivation conditions.DNA Repair (Amst). 2003 Dec 9;2(12):1337-52. doi: 10.1016/j.dnarep.2003.08.002. DNA Repair (Amst). 2003. PMID: 14642563
-
Potential of Photodynamic Therapy Based on Sugar-Conjugated Photosensitizers.J Clin Med. 2021 Feb 18;10(4):841. doi: 10.3390/jcm10040841. J Clin Med. 2021. PMID: 33670714 Free PMC article. Review.
-
Versatile Porphyrin Arrangements for Photodynamic Therapy-A Review.Nanomaterials (Basel). 2024 Nov 22;14(23):1879. doi: 10.3390/nano14231879. Nanomaterials (Basel). 2024. PMID: 39683268 Free PMC article. Review.
Cited by
-
Study and application of the interaction between asymmetrical porphyrin and ascorbic acid.J Fluoresc. 2009 Sep;19(5):809-15. doi: 10.1007/s10895-009-0478-7. Epub 2009 Mar 27. J Fluoresc. 2009. PMID: 19326193
References
-
- Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter BO, Angell-Petersen E, Warloe T, Frandsen N, Hogset A. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc. 2005;218:133–147. - PubMed
-
- Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145–157. - PubMed
-
- Harris F, Sayed Z, Hussain S, Phoenix D. An investigation into the potential of phenothiazinium-based photo-sensitisers to act as PDT agents. Photodiagn and Photodyn Ther. 2004;1:231–239. - PubMed
-
- Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem Photobiol. 1998;68:370–376. - PubMed
-
- Hass BS, Webb RB. Photodynamic effects of dyes on bacteria. IV. Lethal effects of acridine orange and 460- or 500-nm monochromatic light in strains of Escherichia coli that differ in repair capability. Mutat Res. 1981;81:277–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

