Mathematical models of the VEGF receptor and its role in cancer therapy
- PMID: 17251148
- PMCID: PMC2359839
- DOI: 10.1098/rsif.2006.0170
Mathematical models of the VEGF receptor and its role in cancer therapy
Abstract
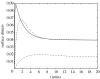
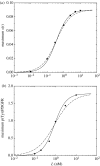
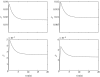
We present an analysis of a stochastic model of the vascular endothelial growth factor (VEGF) receptor. This analysis addresses the contribution of ligand-binding-induced oligomerization, activation of src-homology 2 domain-carrying kinases and receptor internalization in the overall behaviour of the VEGF/VEGF receptor (VEGFR) system. The analysis is based upon a generalization of a Wentzel-Kramers-Brillouin (WKB) approximation of the solution of the corresponding master equation. We predict that tumour-mediated overexpression of VEGFRs in the endothelial cells (ECs) of tumour-engulfed vessels leads to an increased sensitivity of the ECs to low concentrations of VEGF, thus endowing the tumour with increased resistance to anti-angiogenic treatment.
Figures
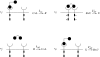











References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Garland Publishing; New York, NY: 2002. Molecular biology of the cell.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

