Diamond and biology
- PMID: 17251162
- PMCID: PMC2373407
- DOI: 10.1098/rsif.2006.0196
Diamond and biology
Abstract
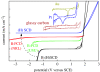
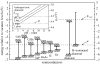
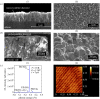
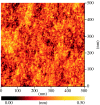
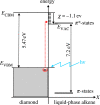
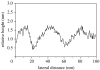
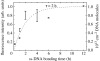
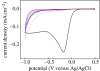
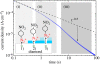
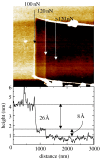
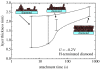
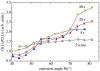
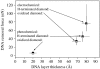
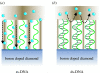
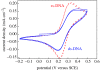
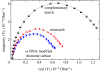
A summary of photo- and electrochemical surface modifications applied on single-crystalline chemical vapour deposition diamond films is given. The covalently bonded formation of amine and phenyl linker molecular layers is characterized using X-ray photoelectron spectroscopy, atomic force microscopy (AFM), cyclic voltammetry and field-effect transistor characterization experiments. Amine and phenyl layers are very different with respect to formation, growth, thickness and molecular arrangement. We deduce a sub-monolayer of amine linker molecules on diamond with approximately 10% coverage of 1.510(15) cm(-2) carbon bonds. Amine is bonded only on initially H-terminated surface areas. In the case of electrochemical deposition of phenyl layers, multilayer properties are detected with three-dimensional nitrophenyl growth properties. This leads to the formation of typically 25 A thick layers. The electrochemical bonding to boron-doped diamond works on H-terminated and oxidized surfaces. After reacting such films with heterobifunctional cross-linker molecules, thiol-modified ss-DNA markers are bonded to the organic system. Application of fluorescence and AFM on hybridized DNA films shows dense arrangements with densities up to 10(13) cm(-2). The DNA is tilted by an angle of approximately 35 degrees with respect to the diamond surface. Shortening the bonding time of thiol-modified ss-DNA to 10 min causes a decrease in DNA density to approximately 10(12) cm(-2). Application of AFM scratching experiments shows threshold removal forces of approximately 75 and 45 nN for the DNA bonded to the phenyl and the amine linker molecules, respectively. First, DNA sensor applications using Fe(CN6) 3-/4- mediator redox molecules and DNA field-effect transistor devices are introduced and discussed.
Figures





















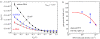

References
-
- Allongue P, Henry de Villeneuve C, Cherouvrier G, Cortes R, Bernard M.-C. Phenyl layers on H–Si(111) by electrochemical reduction of diazonium salts: monolayer versus multilayer formation. J. Electroanal. Chem. 2003;550–551:161–174. doi: 10.1016/S0022-0728(03)00076-7. - DOI
-
- Angus J.C, Pleskov Y.V, Eaton S.C. Electrochemistry of diamond. In: Nebel C.E, Ristein J, editors. Thin film diamond II. Semiconductors and semimetals. vol. 77. Elsevier; Academic Press; Amsterdam, The Netherlands; New York, NY: 2004. p. 97.
-
- Bergonzo P, Jackman R. Diamond-based radiation and photon detectors. In: Nebel C.E, Ristein J, editors. Thin-film diamond I. Semiconductors and semimetals. vol. 76. Elsevier; Amsterdam, The Netherlands: 2004. pp. 197–309.
-
- Bergonzo P, Tromson D, Mer C. Radiation detection devices made from CVD diamond. Semicond. Sci. Technol. 2003;18:S105–S112. doi: 10.1088/0268-1242/18/3/315. - DOI
-
- Borst T.H, Weis O. Boron-doped homoepitaxial diamond layers: fabrication, characterization, and electronic applicationssol. Phys. Status Solidi A. 1996;154:423–444.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
