The Delta intracellular domain mediates TGF-beta/Activin signaling through binding to Smads and has an important bi-directional function in the Notch-Delta signaling pathway
- PMID: 17251195
- PMCID: PMC1807952
- DOI: 10.1093/nar/gkl1128
The Delta intracellular domain mediates TGF-beta/Activin signaling through binding to Smads and has an important bi-directional function in the Notch-Delta signaling pathway
Abstract
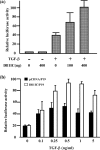
Delta is a major transmembrane ligand for Notch receptor that mediates numerous cell fate decisions. The Notch signaling pathway has long been thought to be mono-directional, because ligands for Notch were generally believed to be unable to transmit signals into the cells expressing them. However, we showed here that Notch also supplies signals to neighboring mouse neural stem cells (NSCs). To investigate the Notch-Delta signaling pathway in a bi-directional manner, we analyzed functional roles of the intracellular domain of mouse Delta like protein 1 (Dll1IC). In developing mouse NSCs, Dll1IC, which is released from cell membrane by proteolysis, is present in the nucleus. Furthermore, we screened for transcription factors that bind to Dll1IC and demonstrated that Dll1IC binds specifically to transcription factors involved in TGF-beta/Activin signaling--Smad2, Smad3 and Smad4--and enhances Smad-dependent transcription. In addition, the results of the present study indicated that over-expression of Dll1IC in embryonic carcinoma P19 cells induced neurons, and this induction was blocked by SB431542, which is a specific inhibitor of TGF-beta/Activin signaling. These observations strongly suggested that Dll1IC mediates TGF-beta/Activin signaling through binding to Smads and plays an important role for bi-directional Notch-Delta signaling pathway.
Figures






Similar articles
-
Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells.Stem Cells. 2010 Apr;28(4):734-42. doi: 10.1002/stem.319. Stem Cells. 2010. PMID: 20146266
-
Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3.J Cell Biol. 2003 Nov 24;163(4):723-8. doi: 10.1083/jcb.200305112. J Cell Biol. 2003. PMID: 14638857 Free PMC article.
-
Modeling and analysis of MH1 domain of Smads and their interaction with promoter DNA sequence motif.J Mol Graph Model. 2009 Apr;27(7):803-12. doi: 10.1016/j.jmgm.2008.12.003. Epub 2008 Dec 24. J Mol Graph Model. 2009. PMID: 19157940
-
Tgf-Beta signaling in development.Sci STKE. 2007 Aug 14;2007(399):cm1. doi: 10.1126/stke.3992007cm1. Sci STKE. 2007. PMID: 17699101 Review.
-
Complex and context dependent regulation of hematopoiesis by TGF-beta superfamily signaling.Ann N Y Acad Sci. 2009 Sep;1176:55-69. doi: 10.1111/j.1749-6632.2009.04569.x. Ann N Y Acad Sci. 2009. PMID: 19796233 Review.
Cited by
-
Lfng regulates the synchronized oscillation of the mouse segmentation clock via trans-repression of Notch signalling.Nat Commun. 2012;3:1141. doi: 10.1038/ncomms2133. Nat Commun. 2012. PMID: 23072809
-
Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4 In Vivo.PLoS Genet. 2015 Jun 26;11(6):e1005328. doi: 10.1371/journal.pgen.1005328. eCollection 2015 Jun. PLoS Genet. 2015. PMID: 26114479 Free PMC article.
-
Transmission of survival signals through Delta-like 1 on activated CD4+ T cells.Sci Rep. 2016 Sep 23;6:33692. doi: 10.1038/srep33692. Sci Rep. 2016. PMID: 27659682 Free PMC article.
-
Normal development in mice over-expressing the intracellular domain of DLL1 argues against reverse signaling by DLL1 in vivo.PLoS One. 2013 Oct 22;8(10):e79050. doi: 10.1371/journal.pone.0079050. eCollection 2013. PLoS One. 2013. PMID: 24167636 Free PMC article.
-
An overview of notch signaling in adult tissue renewal and maintenance.Curr Alzheimer Res. 2012 Feb;9(2):227-40. doi: 10.2174/156720512799361600. Curr Alzheimer Res. 2012. PMID: 21605032 Free PMC article. Review.
References
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Justice NJ, Jan YN. Variations on the Notch pathway in neural development. Curr. Opin. Neurobiol. 2002;12:64–70. - PubMed
-
- Henderson ST, Gao D, Lambie EJ, Kimble J. Lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–2924. - PubMed
-
- Fitzgerald K, Greenwald I. Interchangeability of Caenorhabditis elegans DSL proteins and intrinsic signalling activity of their extracellular domains in vivo. Development. 1995;121:4275–4282. - PubMed
-
- Baker R, Schubiger G. Autonomous and nonautonomous Notch functions for embryonic muscle and epidermis development in Drosophila. Development. 1996;122:617–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

