Inhibition of human immunodeficiency virus type 1 infection of human CD4+ T cells by microbial HSP70 and the peptide epitope 407-426
- PMID: 17251296
- PMCID: PMC1866028
- DOI: 10.1128/JVI.02320-06
Inhibition of human immunodeficiency virus type 1 infection of human CD4+ T cells by microbial HSP70 and the peptide epitope 407-426
Abstract
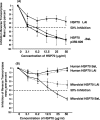
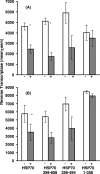
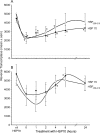
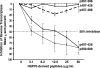
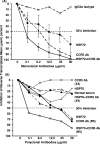
Human immunodeficiency virus type 1 (HIV-1) virions contain heat shock proteins (HSP), but these proteins have received limited attention. The objectives of this study were to establish if the microbial 70-kDa HSP exerts an inhibitory effect on the HIV-1 infection of human CD4+ T cells, to identify an inhibitory peptide epitope within the sequence of HSP70, and to evaluate the kinetic features of any inhibitory activity. The results of these studies suggest that microbial HSP70 exerts dose-dependent inhibition on CCR5 (R5) strains of clades B, C, and D of HIV-1 infecting human CD4+ T cells. The site of the HIV-1-inhibitory function was identified within the C-terminal peptide binding domain of HSP70, and the function is expressed by the peptide epitope comprising amino acids 407 to 426. The mechanism of inhibition of HIV-1 infectivity by HSP70 is blocking of the CCR5 coreceptors directly and indirectly by inducing CC chemokines and APOBEC3G. The inhibitory effect of HSP70, its C-terminal fragment, or peptide 407-426 may make HSP70 useful as a microbicidal agent. A potentiating noncognate inhibition of HIV-1 infectivity by combined treatment with HSP70 and monoclonal or polyclonal antibody to CCR5 was demonstrated. This novel strategy may be utilized in therapeutic immunization against HIV-1 infection.
Figures
References
-
- Agnew, L. L., M. Kelly, J. Howard, S. Jeganathan, M. Batterham, M. A. French, J. Gold, and K. Watson. 2003. Altered lymphocyte heat shock protein 70 expression in patients with HIV disease. AIDS 17:1985-1988. - PubMed
-
- Agostini, I., L. S. Popov, J. Li, L. Dubrovsky, T. Hao, and M. Bukrinsky. 2000. Heat-shock protein 70 can replace viral protein R of HOV-1 during nuclear import of the viral preintegration complex. Exp. Eye Res. 259:398-403. - PubMed
-
- Barouch, D. H., et al. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. - PubMed
-
- Bogers, W. M. J. M., L. A. Bergmeier, J. Ma, H. Oostermeijer, Y. Wang, C. G. Kelly, P. ten Haaft, M. Singh, J. L. Heeney, and T. Lehner. 2004. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. AIDS 18:25-36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

