GABAergic spill-over transmission onto hippocampal mossy fiber boutons
- PMID: 17251436
- PMCID: PMC6672922
- DOI: 10.1523/JNEUROSCI.4996-06.2007
GABAergic spill-over transmission onto hippocampal mossy fiber boutons
Abstract
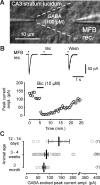
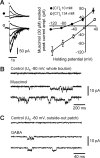
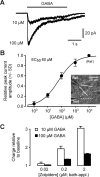
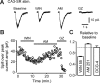
Presynaptic ionotropic GABA(A) receptors have been suggested to contribute to the regulation of cortical glutamatergic synaptic transmission. Here, we analyzed presynaptic GABA(A) receptor-mediated currents (34 degrees C) recorded from mossy fiber boutons (MFBs) in rat hippocampal slices. In MFBs from young and adult animals, GABA puff application activated currents that were blocked by GABA(A) receptor antagonists. The conductance density of 0.65 mS x cm2 was comparable to that of other presynaptic terminals. The single-channel conductance was 36 pS (symmetrical chloride), yielding an estimated GABA(A) receptor density of 20-200 receptors per MFB. Presynaptic GABA(A) receptors likely contain alpha2-subunits as indicated by their zolpidem sensitivity. In accordance with the low apparent GABA affinity (EC50 = 60 microM) of the receptors and a tight control of ambient GABA concentration by GABA transporters, no tonic background activation of presynaptic GABA(A) receptors was observed. Instead, extracellular high-frequency stimulation led to transient presynaptic currents, which were blocked by GABA(A) receptor antagonists but were enhanced by block of GAT 1 (GABA transporter 1), indicating that these currents were generated by GABA spill-over and subsequent presynaptic GABA(A) receptor activation. Presynaptic spill-over currents were depressed by pharmacological cannabinoid 1 (CB1) receptor activation, suggesting that GABA was released predominantly by a CB1 receptor-expressing interneuron subpopulation. Because GABA(A) receptors in axons are considered to act depolarizing, high activity of CB1 receptor-expressing interneurons will exert substantial impact on presynaptic membrane potential, thus modulating action potential-evoked transmitter release at the mossy fiber-CA3 synapse.
Figures


References
-
- Alger BE, Nicoll RA. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979;281:315–317. - PubMed
-
- Alle H, Geiger JRP. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
