Structure-function relations of human hemoglobins
- PMID: 17252042
- PMCID: PMC1484532
- DOI: 10.1080/08998280.2006.11928171
Structure-function relations of human hemoglobins
Abstract
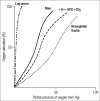
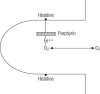
In 1949 Pauling and his associates showed that sickle cell hemoglobin (HbS) belonged to an abnormal molecular species. In 1958 Ingram, who used a two-dimensional system of electrophoresis and chromatography to break down the hemoglobin molecule into a mixture of smaller peptides, defined the molecular defect in HbS by showing that it differed from normal adult hemoglobin by only a single peptide. Since then, more than 200 variant and abnormal hemoglobins have been described. Furthermore, the construction of an atomic model of the hemoglobin molecule based on a high-resolution x-ray analysis by Dr. Max Perutz at Cambridge has permitted the study of the stereochemical part played by the amino acid residues, which were replaced, deleted, or added to in each of the hemoglobin variants. Some of the variants have been associated with clinical conditions. The demonstration of a molecular basis for a disease was a significant turning point in medicine. A new engineered hemoglobin derived from crocodile blood, with markedly reduced oxygen affinity and increased oxygen delivery to the tissues, points the way for future advances in medicine.
Figures

References
-
- Perutz MF, Rossmann MG, Cullis MG, Muirhead H, Will G, North ACT Structure of haemoglobin. A three-dimensional Fourier synthesis at 5.5Å resolution, obtained by X-ray analysis. Nature. 1960;(185):416–422. - PubMed
-
- Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;(180):326–328. - PubMed
-
- Perutz MF, Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968;219(157):902–909. - PubMed
-
- Marengo-Rowe A. Haemoglobinopathies. Br J Hosp Med. 1971;6:617–630.
LinkOut - more resources
Full Text Sources
Other Literature Sources
