Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid
- PMID: 17253458
- PMCID: PMC10849111
- DOI: 10.1002/14651858.CD001385.pub2
Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid
Abstract
Background: Asthma is a common respiratory disease among both adults and children and short acting inhaled beta-2 agonists are used widely for 'reliever' bronchodilator therapy. Long acting beta-2 agonists (LABA) were introduced as prospective 'symptom controllers' in addition to inhaled corticosteroid 'preventer' therapy (ICS). In this updated review we have included studies in which patients were either not on ICS as a group, or in which some patients, but not all, were on ICS to complement previous systematic reviews of studies where LABA was given in patients uniformly receiving ICS. We have focussed particularly on serious adverse events, given previous concerns about potential risks, especially of death, from regular beta-2 agonist use.
Objectives: This review aimed to determine the benefit or detriment on the primary outcome of asthma control with the regular use of LABA compared with placebo, in mixed populations in which only some were taking ICS and in populations not using ICS therapy.
Search strategy: We carried out searches using the Cochrane Airways Group trial register, most recently in October 2005. We searched bibliographies of identified RCTs for additional relevant RCTs and contacted authors of identified RCTs for other published and unpublished studies.
Selection criteria: All randomised studies of at least four weeks duration, comparing a LABA given twice daily with a placebo, in chronic asthma. Selection criteria to this updated review have been altered to accommodate recently published Cochrane reviews on combination and addition of LABA to ICS therapy. Studies in which all individuals were uniformly taking ICS were excluded from this review.
Data collection and analysis: Two reviewers performed data extraction and study quality assessment independently. We contacted authors of studies for missing data.
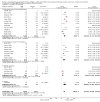
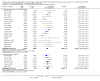
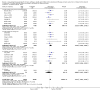
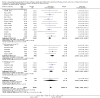
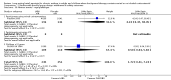
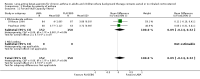
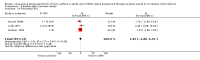
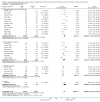
Main results: Sixty-seven studies (representing 68 experimental comparisons) randomising 42,333 participants met the inclusion criteria. Salmeterol was used as long-acting agent in 50 studies and formoterol fumarate in 17. The treatment period was four to nine weeks in 29 studies, and 12 to 52 weeks in 38 studies. Twenty-four studies did not permit the use of ICS, and forty permitted either inhaled corticosteroid or cromones (in three studies this was unclear). In these studies between 22% and 92% were taking ICS, with a median of 62%. There were significant advantages to LABA treatment compared to placebo for a variety of measurements of airway calibre including morning peak expiratory flow (PEF), evening PEF and FEV1. They were associated with significantly fewer symptoms, less use of rescue medication and higher quality of life scores. This was true whether patients were taking LABA in combination with ICS or not. Findings from SMART (a recently published surveillance study) indicated significant increases in asthma related deaths, respiratory related deaths and combined asthma related deaths and life threatening experiences. The absolute increase in asthma-related mortality was consistent with an increase of around one per 1250 patients treated with LABA for six months, but the confidence intervals are wide (from 700 to 10,000). Post-hoc exploratory subgroups suggested that African-Americans and those not on inhaled corticosteroids were at particular risk for the primary end-point of death or life-threatening asthma event. There was also a suggestion of an increase in exacerbation rate in children. Pharmacologically predicted side effects such as headache, throat irritation, tremor and nervousness were more frequent with LABA treatment.
Authors' conclusions: LABA are effective in the control of chronic asthma in the "real-life" subject groups included. However there are potential safety issues which call into question the safety of LABA, particularly in those asthmatics who are not taking ICS, and it is not clear why African-Americans were found to have significant differences in comparison to Caucasians for combined respiratory-related death and life threatening experiences, but not for asthma-related death.
Conflict of interest statement
E H Walters has taken part in collaborative clinical pharmacology studies with a number of pharmaceutical companies including GSK, Astra Zeneca, Pfizer, Boehringer, Schering Plough, SKB, and Novartis. He has, in the past, held consultancies with GSK, Pfizer and Zeneca. He has had sponsorship to meetings from a number of the companies listed over the past 15 years. PG Gibson has taken part in collaborative clinical studies with a number of pharmaceutical companies including GSK, AstraZeneca, Boerhinger Ingelheim, and Aventis. He has attended meetings sponsored by a number of companies.
Figures


























































































































































































































Update of
-
Inhaled long acting beta agonists for stable chronic asthma.Cochrane Database Syst Rev. 2003;(4):CD001385. doi: 10.1002/14651858.CD001385. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2007 Jan 24;(1):CD001385. doi: 10.1002/14651858.CD001385.pub2. PMID: 14583933 Updated.
References
References to studies included in this review
Adinoff 1998 {published data only}
-
- Adinoff A, Schwartz H, Rickard K, Yancey S, Swearingen B. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47(4):278‐84. - PubMed
Bensch 2001 {published data only}
-
- Bensch G, Lapidus R, Levine B, Lumry W, Yegen U, Kiselev P, et al. A randomized, 12‐week, double‐blind, placebo‐controlled study comparing formoterol dry powder inhaler with albuterol metered‐dose inhaler. Annals of Allergy Asthma & Immunology 2001;86(1):19‐27. - PubMed
-
- Della Cioppa G, Kiseley P, Matos D, Yegen I, Townley RG. QID Albuterol worsens peak flow variability in asthma whereas BID Formoterol does not [abstract]. Annals of Allergy 1998;80:88.
-
- FORNDA 20831_40. A twelve week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered dose inhaler device versus placebo in patients with mild to moderate asthma. www.fda.gov 2001.
-
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003;124(1):70‐4. - PubMed
Bensch 2002 {published data only}
-
- Bensch G, Berger WE, Blokhin BM, Socolovsky AL, Thomson MH, Till MD, et al. One‐year efficacy and safety of inhaled formoterol dry powder in children with persistent asthma. Annals of Allergy, Asthma, & Immunology 2002;89(2):180‐90. - PubMed
-
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003;124(1):70‐4. - PubMed
Booth 1993 {published data only}
Boulet 1997 {published data only}
-
- Boulet LP, Laviolette M, Boucher S, et al. A Twelve week comparison of salmeterol and salbutamol in the treatment of mild to moderate asthma‐ a Canadian multicentre study. Journal of Allergy & Clinical Immunology 1997;99(1 Pt 1):13‐21. - PubMed
Boulet 1998b {published data only}
-
- Boulet L, Cartier A, Milot J, Cote J, Malo JL, Laviolette M. Tolerance to the protective effects of salmeterol on methacholine‐induced bronchoconstriction‐ influence of inhaled corticosteroids. European Respiratory Journal 1998;11(5):1091‐7. - PubMed
Busse 1998 {published data only}
-
- Busse WW, Casale TB, Murray JJ, Petrocella V, Cox F, Rickard K. Efficacy, safety, and impact on quality of life of salmeterol in patients with moderate persistent asthma. American Journal of Managed Care 1998;4(11):1579‐87. - PubMed
Busse 2004 {published data only}
-
- Busse W, Levine B, Andriano K, Lavecchia C, Yegen U. Efficacy, tolerability, and effect on asthma‐related quality of life of formoterol bid via multidose dry powder inhaler and albuterol QID via metered dose inhaler in patients with persistent asthma: a multicenter, randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group study. Clnical Therapeutics 2004;26(10):1587‐98. - PubMed
Cheung 1992 {published data only}
-
- Cheung D, Timmers MC, Zwinderman AH, et al. Long term effects of a long acting beta adrenoceptor agonist in patients with mild asthma. New England Journal of Medicine 1992;327(17):1198‐1203. - PubMed
Cloostermann2001 {published data only}
-
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta(2)‐agonists in allergic asthmatic patients. Chest 2001;119(5):1306‐15. - PubMed
-
- Cloosterman SG, Schermer TR, Bijl‐Hofland ID, Heide S, Brunekreef B, Elshout FJ, et al. Effects of house dust mite avoidance measures on Der p 1 concentrations and clinical condition of mild adult house dust mite‐allergic asthmatic patients, using no inhaled steroids. Clinical & Experimental Allergy 1999;29(10):1336‐46. - PubMed
-
- Schayck CP, Cloosterman SG, Bijl‐Hofland ID, Hoogen H, Folgering HT, Weel C. Is the increase in bronchial responsiveness or FEV1 shortly after cessation of beta2‐agonists reflecting a real deterioration of the disease in allergic asthmatic patients? A comparison between short‐acting and long‐acting beta2‐agonists. Respiratory Medicine 2002;96(3):155‐62. - PubMed
Creticos 1999 {published data only}
-
- Creticos PS, Freidhoff LR, Bernstein DI, Chu T, Khattignavong AP, Pasatiempo AM, et al. Comparison of an inhaled corticosteroid (triamcinolone acetonide) to a long‐acting bronchodilator (salmeterol), the combination, and placebo in mild‐moderate adult asthmatic patients. International Archives of Allergy and Immunology 1999;118(2‐4):345‐6. - PubMed
D'Alonzo 1994 {published data only}
-
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy & Clinical Immunology 1996;98(6 Pt 1):1116‐9. - PubMed
-
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5:128‐32.
-
- D'Alonzo GE, Nathan RA, Henchowicz MD. Salmeterol xinafoate as maintenance therapy compared with albuterol in patients with asthma. JAMA 1994;271(18):1412‐6. - PubMed
-
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma, & Immunology 1995;75(3):243‐8. - PubMed
Dahl 1991a {published data only}
-
- Dahl R, Earnshaw JS, Palmer JBD. Salmeterol‐a 4 week study of a long acting beta2 adrenoceptor agonist for the treatment of reversible airways disease. European Respiratory Journal 1991;4(10):1178‐84. - PubMed
-
- Faurschou P. Chronic dose‐ranging studies with salmeterol. European Respiratory Review 1991;1(4):282‐7.
Dahl 1991b {published data only}
-
- Dahl R, Pedersen B, Venge P. Bronchoalveolar lavage studies. European Respiratory Review 1991;1:272‐5.
Ekstrom 1998a {published data only}
-
- Ekstrom T, Ringdal N, Sobradillo V, Runnerstrom E, Soliman S. Low‐dose formoterol Turbuhaler(TM) (Oxis(TM)) bid, a 3‐month placebo‐controlled comparison with terbutaline (qid). Respiratory Medicine 1998;92(8):1040‐5. - PubMed
Ekstrom 1998b {published data only}
-
- Ekstrom T, Ringdal N, Tukiainen P, Runnerstrom E, Soliman S. A 3 month comparison of formoterol with terbutaline via turbuhaler‐A placebo controlled study. Annals of Allergy, Asthma, & Immunology 1998;81(3):225‐30. - PubMed
Garcia 2001 {published data only}
-
- Garcia R, Guerra P, Feo F, Galindo P, Gomez E, Borja J, et al. Tachyphylaxis following regular use of formoterol in exercise‐induced bronchospasm. Journal of Investigational Allergology & Clinical Immunology 2001;11(3):176‐82. - PubMed
Hyland 1994 {published data only}
-
- Hyland ME, Kenyon CAP, Jacobs P. Sensitivity of quality of life domains and constructs to longitudinal change in a clinical trial comparing salmeterol and placebo in asthmatics. Quality of Life Research 1994;3(2):121‐6. - PubMed
Jones 1994 {published data only}
Juniper 1995 {published data only}
-
- Juniper EF, Johnston P, Borkhoff C, Haukioja A. A multicentre comparison of salmeterol and salbutamol on asthma‐specific quality of life [abstract]. Quality of Life Research 1994;3:83.
-
- Juniper EF, Johnston PR, Borkhoff CM, Guyatt G, Boulet L‐P, Haukioja A. Quality of life in asthma clinical trials‐a comparison of salmeterol and salbutamol. American Journal of Respiratory & Critical Care Medicine 1995;151(1):66‐70. - PubMed
Kavuru 2000a {published data only}
-
- Edwards T, Gross G, Mitchell D, Chervinsky P, Woodring A, Baitinger L, et al. The salmeterol xinafoate/fluticasone propionate dry powder combination product via diskus(r) inhaler improves asthma control compared to salmeterol xinafoate or fluticasone propionate dry powder alone. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A414.
-
- Kavuru M, Melamed J, Gross G, LaForce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma‐A randomized, double‐blind, placebo‐controlled trial. Journal of Allergy & Clinical Immunology 2000;105(6):1108‐16. - PubMed
-
- SFCA3002. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of salmeterol 50mcg BID and fluticasone propionate 100mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk 2005.
Kemp 1998a {published data only}
-
- Kemp J, Wolfe J, Grady J, LaForce C, Stahl E, Arlidge T, et al. Salmeterol powder compared with albuterol aerosol as maintenance therapy for asthma in adolescent and adult patients. Clinical Therapeutics 1998;20(2):270‐82. - PubMed
-
- Wolfe J, LaForce C, Chervinsky P, Galant S, Lumry W, Noonan M, et al. Inhaled salmeterol powder compared with albuterol aerosol given regularly or as needed for asthma (abstract). Annals of Allergy, Asthma and Immunology 1995;74:52.
Kemp 1999 {published data only}
-
- Chervinsky P, Goldberg P, Galant S, Wang Y, Arledge T, Welch MB, et al. Long‐term cardiovascular safety of salmeterol powder pharmacotherapy in adolescent and adult patients with chronic persistent asthma‐A randomized clinical trial. Chest 1999;115(3):642‐8. - PubMed
-
- Kemp J, DeGraff A, Pearlman D, Chervinsky P, Galant S, Goldberg P, et al. A 1‐year study of salmeterol powder on pulmonary function and hyperresponsiveness to methacholine. Journal of Allergy & Clinical Immunology 1999;104(6):1189‐97. - PubMed
-
- Rosenthal R, Chervinsky P, DeGraaf A, et al. Long term regular treatment with salmeterol is effective in protecting against bronchial hyperresponsiveness as measured by methacholine challenge in asthmatics. Journal of Allergy and Clinical Immunology 1997;99(1 Pt 2):323s.
Kraft 1997 {published data only}
-
- Kraft M, Wenzel SE, Bettinger CM, Martin RJ. The effect of salmeterol on nocturnal symptoms, airway function and inflammation in asthma. Chest 1997;111(5):1249‐54. - PubMed
LaForce 2005 {published data only}
-
- LaForce C, Prenner BM, Andriano K, Lavecchia C, Yegen U. Efficacy and safety of formoterol delivered via a new multidose dry powder inhaler (Certihaler) in adolescents and adults with persistent asthma. Journal of Asthma 2005;42(2):101‐6. - PubMed
Lazarus 2001 {published data only}
-
- Lazarus SC, Boushey H, Fahy JV, Chinchilli VM, Lemanske RF, Sorkness CA, et al. Long‐acting ß2‐agonist monotherapy versus continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. Journal of the American Medical Association 2001;285(20):2583‐93. - PubMed
Leblanc 1996 {published data only}
-
- Leblanc P, Knight A, Kreisman H, Borkhoff CM, Johnston PR. A placebo controlled crossover comparison of salmeterol and salbutamol in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1996;154(2 Pt 1):324‐8. - PubMed
Levy 2005 {published data only}
-
- Levy R, Pinnas J, Milgrom H, Smith J, Yegen U. Safety and efficacy in children with persistent asthma treated with formoterol 10 mcg BID delivered via Certihaler: a novel multidose dry‐powder inhaler. Pediatric Asthma, Allergy, and Immunology 2005;18(1):25‐35.
Lindqvist 2003 {published and unpublished data}
-
- Lindqvist A, Karjalainen EM, Laitinen LA, Kava T, Altraja A, Pulkkinen M, et al. Salmeterol resolves airway obstruction but does not possess anti‐eosinophil efficacy in newly diagnosed asthma: a randomized, double‐blind, parallel group biopsy study comparing the effects of salmeterol, fluticasone propionate, and disodium cromoglycate. Journal of Allergy & Clinical Immunology 2003;112(1):23‐8. - PubMed
Lockey 1999 {published data only}
-
- Lockey R, DuBuske L, Friedman B, Petrocella V, Cox F, Rickard K. Nocturnal Asthma. Effect of Salmeterol on Quality of Life and Clinical Outcomes. Chest 1999;115(3):666‐73. - PubMed
Mahajan 1998 {published data only}
-
- Mahajan P, Stahl E, Arledge T. Quality of life in pediatric asthma patients treated with salmeterol and impact on the daily activities of their parents. Pediatric Asthma Allergy & Immunology 1998;12(1):21‐8.
Nathan 1999 {published data only}
-
- Nathan R, Pinnas J, Schwartz H, Grossman J, Yancey S, Emmett A, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy 1999;82(6):521‐9. - PubMed
Nelson 1998 {published data only}
-
- Nelson J, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden EJ. Effect of long‐term salmeterol treatment on exercise‐induced asthma. New England Journal of Medicine 1998;339(3):141‐6. - PubMed
Nelson 1999b {published data only}
-
- Nelson H, Berkowitz R, Tinkelman D, Emmett A, Rickard K, Yancey S. Lack of Subsensitivity to Albuterol After Treatment with Salmeterol in Patients with Asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(5 Pt 1):1556‐61. - PubMed
-
- SLGA5020. A single‐center, randomized, double‐blind, cross‐over clinical trial to examine sub‐sensitivity in adult subjects with asthma receiving salmeterol xinafoate 42mcg BID and placebo BID. www.ctr.gsk.co.uk.
Newnham 1994 {published data only}
-
- Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator sub‐sensitivity after chronic dosing with eformoterol in patients with asthma. American Journal of Medicine 1994;97(1):29‐37. - PubMed
Newnham 1995 {published data only}
-
- Tan KS, Hall IP, Dewar JC, Dow E, Lipworth BJ. Association between beta2‐adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet 1997;350(9083):995‐9. - PubMed
Pearlman 1992 {published data only}
-
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy & Clinical Immunology 1996;98(6 Pt 1):1116‐9. - PubMed
-
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma, & Immunology 1995;75(3):243‐8. - PubMed
-
- Pearlman DS. Long‐acting beta 2‐agonist salmeterol compared with albuterol in maintenance asthma therapy. Annals of Allergy Asthma & Immunology 1995;75(2):180‐4. - PubMed
-
- Pearlman DS, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild‐to‐moderate asthma. New England Journal Of Medicine 1992;327(20):1420‐5. - PubMed
-
- Pearlman DS, Liddle R. Controlling asthma symptoms: Salmeterol compared with salbutamol in large‐scale multicentre studies. European Respiratory Review 1994;4(21):301‐5.
Pearlman 1999a {published data only}
-
- Pearlman D, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: A study comparing monotherapy and combination therapy in asthma. Annals of Allergy 1999;82(3):257‐65. - PubMed
Pleskow 2003 {published data only}
-
- FORNDA 20831_41. A twelve week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered dose inhaler device versus placebo in patients with mild to moderate asthma. www.fda.gov 2001.
-
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003;124(1):70‐4. - PubMed
-
- Pleskow W, LaForce C, Yegen Ü, Matos D, Della Cioppa G. Formoterol Delivered via the Dry Powder Aerolizer® Inhaler Versus Albuterol MDI and Placebo in Mild‐to‐Moderate Asthma: A Randomized, Double‐Blind, Double‐Dummy Trial. Journal of Asthma 2003;40(5):505‐14. - PubMed
Prieto 2002 {published data only}
-
- Prieto L, Gutierrez V, Torres V, Uixera S, Marin J. Effect of salmeterol on seasonal changes in airway responsiveness and exhaled nitric oxide in pollen‐sensitive asthmatic subjects. Chest 2002;122(3):798‐805. - PubMed
Ramage 1994 {published data only}
-
- Ramage L, Cree IA, Dhillon DP. Comparison of salmeterol with placebo in mild asthma: effect on peripheral blood phagocyte function and cytokine levels. International Archives of Allergy & Immunology 1994;105(2):181‐4. - PubMed
-
- Ramage L, Lipworth BJ, Ingram CG, et al. Reduced protection against exercise induced bronchoconstriction after chronic dosing with salmeterol. Respiratory Medicine 1994;88(5):363‐8. - PubMed
Roberts 1999 {published data only}
-
- Roberts B, Bradding P, Holgate S. Effects of a six week course of salmeterol on bronchial reactivity. Thorax 1992;47:230P.
-
- Roberts J, Bradding P, Britten K, Walls A, Wilson S, Gratziou C, et al. The long‐acting beta2‐agonist salmeterol xinafoate: effects on airway inflammation in asthma. European Respiratory Journal 1999;14(2):275‐82. - PubMed
Rosenthal 1999 {published data only}
-
- Rosenthal R, Busse W, Kemp J, Baker J, Kalberg C, Emmett A, et al. Effect of long‐term salmeterol therapy compared with as‐needed albuterol use on airway hyperresponsiveness. Chest 1999;116(3):595‐602. - PubMed
SAS30003 {unpublished data only}
-
- SAS30003. A stratified, randomized, double‐blind, placebo‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in HFA 134a MDI, 42/88mcg BID, and salmeterol in propellant 11/12 MDI, 42mcg BID, fluticasone propionate in propellant 11/12 MDI, 88mcg BID, and placebo propellant HFA 134a MDI in adult and adolescent subjects with asthma. www.ctr.gsk.co.uk.
SAS30004 {published data only}
-
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life. Journal of Allergy & Clinical Immunology 2001;107(2):S246.
-
- Nathan RA, Mitchell D, Condemi J, Heller A, Schoaf L, Herrle M, et al. Cardiovascular and hypothalamic‐pituitary‐adrenal axis safety of fluticasone propionate/salmeterol HFA MDI in adolescent and adult patients with asthma. American Journal for Respiratory and Critical Care Medicine 2001;163(5):A863.
-
- Pearlman DS, Kent E, Lanz MJ, Peden D, Baitinger L, Herrle M, et al. Fluticasone propionate/salmeterol HFA MDI has a rapid onset of effect in asthmatics treated with short or long‐acting beta‐agonists (BA) or inhaled corticosteroids (ICS). Amercian Journal of Respiratory and Critical Care Medicine 2001;163(5):A865.
-
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The fluticasone propionate/salmeterol HFA MDI is significantly more efficacious in treating asthma than placebo HFA MDI, fluticasone propionate CFC MDI or salmeterol CFC MDI. Journal of Allergy and Clinicial Immunology 2001;107(2):100s.
-
- SAS30004. A randomized, double‐blind, placebo‐controlled, parallel‐group 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in GR106642X MDI, 50/250mcg BID, and salmeterol in propellant 11/12 MDI, 50mcg BID, fluticasone propionate in propellant 11/12 MDI, 250mcg BID, and placebo propellant GR106642X MDI in adult and adolescent subjects with asthma. www.ctr.gsk.co.uk 2004.
Schreurs 1996 {published data only}
-
- Schreurs A, Sinninghe Damste H, Graaff C, Greefhorst A. A dose‐response study with formoterol Turbuhaler as maintenance therapy in asthmatic patients. European Respiratory Journal 1996;9(8):1678‐83. - PubMed
Shapiro 2000b {published data only}
-
- Aggarwal SK, Frith LJ, Ho M, Weeks T, Ho SY. Fluticasone propionate/salmeterol delivered from a single inhaler demonstrates synergistic benefits in asthma. Amercian Journal of Respiratory and Critical Care Medicine 2003;167(7):A890.
-
- Bateman ED, Frith L, Braunstein GL. Achieving guideline‐based asthma control ‐ does the patient benefit?. European Respiratory Journal 2002;20(3):588‐95. - PubMed
-
- Bateman ED, Frith L, Ho M. Guideline‐based asthma control reduces maximally the impact of upon patient‐assessed quality of life. Amercian Journal of Respiratory and Critical Care Medicine 2002;165(8):A44.
-
- Cook CK, Prillaman BA, House KW, Rickard KA, Shah TP. Concurrent use of salmeterol/fluticasone propionate Diskus powder combination product and fluticasone propionate aqueous nasal spray does not adversely affect HPA‐axis function. Annals of Allergy, Asthma and Immunology 2001;86(1):98.
-
- Dorinsky P, Yancey S, Kral K, Emmett A, House K, Prillaman B, et al. Asthma control with salmeterol/fluticasone propionate (50/250mcg) dry powder combination Diskus has a faster onset of effect compared with salmeterol or fluticasone propionate in patients with asthma. Journal Allergy & Clinical Immunology 2000;161(Suppl 1):A195.
Simons 1997a {published data only}
-
- Malozowski S, Stadel BV, Pian LP. Comparison of beclomethasone, salmeterol, and placebo in children with asthma. New England Journal of Medicine 1998; Vol. 339, issue 10:704‐5. - PubMed
-
- Simons FER. A comparison of Beclomethasone, salmeterol and placebo in children with asthma. New England Journal of Medicine 1997;337(23):1659‐65. - PubMed
-
- Simons FER, et al. A one year placebo controlled study of the efficacy and safety of beclomethasone DP versus salmeterol in glucocorticoid naive children with asthma. Journal of Allergy & Clinical Immunology 1997;99:S402.
SLGA2004 {unpublished data only}
-
- SLGA2004. A randomized, double‐blind, double‐dummy, placebo‐controlled, comparative clinical trial of salmeterol xinafoate via multi‐dose powder inhaler versus salmeterol xinafoate via Diskhaler for four weeks in adolescent and adult subjects with mild‐to‐moderate asthma.. http://ctr.gsk.co.uk 2006.
SLGA3014 1994 {unpublished data only}
-
- SLGA3014. www.fda.gov 2001.
SLGL82 {published data only}
-
- SLGL82. A double‐blind parallel group study of inhaled salmeterol in asthmatic patients. www.ctr.gsk.co.uk 2006.
SLMP03 {unpublished data only}
-
- SLMP03. A single centre, randomised, double‐blind, parallel group, placebo controlled study, to evaluate the effect of inhaled salmeterol xinafoate (50micrograms bd from a Diskhaler) on variations in bronchoconstriction induced by methacholine, in paediatric patients with mild to moderate asthma. www.ctr.gsk.co.uk 2006.
SMART {published data only}
-
- Knobil K, Yancey S, Kral K, Rickard K. Salmeterol Multicentre Asthma Research Trial (SMART): Results from an interim analysis. Chest 2003;124:335s.
-
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: A Comparison of Usual Pharmacotherapy for Asthma or Usual Pharmacotherapy Plus Salmeterol. Chest 2006;129(1):15‐26. - PubMed
-
- SLGA5011 SMART. SMART: a double‐blind, randomized, placebo‐controlled surveillance study of asthma event outcomes in subjects receiving either usual pharmacotherapy of asthma or usual pharmacotherapy plus salmeterol 42mcg twice daily. http://www.ctr.gsk.co.uk 2006.
SMS40221 {unpublished data only}
-
- SMS40221. Comparing the efficacy and safety of inhaled salmeterol 50mcg BID and placebo via diskus in patients with reversible airways obstruction. www.ctr.gsk.co.uk 2005.
Starke 1996 {published data only}
-
- Starke I, Luce P. The efficacy and safety of inhaled salmeterol 50 microg bd in older patients with reversible airflow obstruction. Age & Ageing 1996;25(1):67‐71. - PubMed
Steffensen 1995 {published data only}
-
- Steffensen I, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskrift for Laeger 1996;158(49):7092‐6. - PubMed
-
- Steffensen I, Faurschou P, Riska H, Rostrup J, Wegener T. Inhaled formoterol dry powder in the treatment of patients with reversible obstructive airway disease. A 3‐month, placebo‐controlled comparison of the efficacy and safety of formoterol and salbutamol, followed by a 12‐month trial with formoterol. Allergy 1995;50(8):657‐63. - PubMed
Stelmach 2002 {published data only}
-
- Stelmach I, Gorski P, Jerzynska J, Stelmach W, Majak P, Kuna P. A randomized, double‐blind trial of the effect of treatment with formoterol on clinical and inflammatory parameters of asthma in children. Annals of Allergy, Asthma, & Immunology 2002;89(1):67‐73. - PubMed
Sussman 1995 {published data only}
-
- Sussman HS. Continuous eformoterol and beta 2‐receptor responsiveness. British Journal of Clinical Practice 1995;81 Suppl:16‐7. - PubMed
Taylor 1998 {published data only}
von Berg 1998 {published data only}
-
- Berg A, Blic J, Rosa M, et al. A comparison of regular salmeterol vs 'as required' salbutamol therapy in asthmatic children. Respiratory Medicine 1998;92(2):292‐9. - PubMed
-
- Berg A, Blic J, Rosa M, et al. Regular salbutamol xinafoate compared with salbutamol as required in children with moderate asthma. American Journal of Respiratory & Critical Care Medicine 1996;4:A76.
von Berg 2003 {published data only}
-
- Berg A, Papageorgiou Saxoni F, Wille S, Carrillo T, Kattamis C, Helms PJ. Efficacy and tolerability of formoterol Turbuhaler in children. International Journal of Clinical Practice 2003;57(10):852‐6. - PubMed
Wallin 1999 {published data only}
-
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Lundback B, Della‐Cioppa G, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(1):79‐86. - PubMed
Weinstein 1998 {published data only}
-
- Pearlman DS, Bronsky E, Chervinsky P, et al. Inhaled salmeterol powder compared with placebo administered over 12 weeks to children with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1996;153(4):A76.
-
- SLD390. www.fda.gov 2001.
-
- Weinstein S, Pearlman D, Bronsky E, Byrne A, Arledge T, Liddle R, et al. Efficacy of salmeterol xinafoate powder in children with chronic persistent asthma. Annals of Allergy, Asthma, & Immunology 1998;81(1):51‐8. - PubMed
Wolfe 2000a {published and unpublished data}
-
- SLGA3010. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50mcg via the Diskus and salmeterol 50mcg via the metered‐dose inhaler versus placebo for 12 weeks in adolescent and adult subjects with mild‐to‐moderate asthma. www.ctr.gsk.co.uk.
-
- Wolfe J, Kreitzer S, Chervinsky P, Lawrence M, Wang Y, Reilly D, et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Annals of Allergy 2000;84(3):334‐40. - PubMed
Wolfe 2000b {published and unpublished data}
-
- SLGA3011. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50mcg via the Diskus and salmeterol 50mcg via the metered‐dose inhaler versus placebo for twelve weeks in adolescent and adult subjects with mild‐to‐moderate asthma.. www.ctr.gsk.co.uk.
-
- Wolfe J, Kreitzer S, Chervinsky P, Lawrence M, Wang Y, Reilly D, et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Annals of Allergy 2000;84(3):334‐40. - PubMed
Wolfe 2006 {published data only}
-
- FOR258D2307. www.fda.gov 2004.
-
- Lapidus RJ, Wolfe J, Greos LS, Friedman, Orevillo C, Ziehmer B, et al. Formoterol 24 mcg provides effective bronchodilation and is well tolerated in patients with persistent stable asthma [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S150.
-
- Wolfe J, Laforce C, Friedman B, Sokol W, Till D, Della Cioppa G, et al. Formoterol, 24 microg bid, and serious asthma exacerbations: similar rates compared with formoterol, 12 microg bid, with and without extra doses taken on demand, and placebo. Chest 2006;129(1):27‐38. - PubMed
Wronska 1998 {published data only}
-
- Wronska J, Chazan R, Mazurek J, Droszcz W. Treatment with salmeterol and quality of life in patients with asthma. Pneumonologia i Alergologia Polska 1998;66(3‐4):193‐7. - PubMed
Zarkovic 1998 {published data only}
-
- Gotz MH, Taak NK. The efficacy and safety of inhaled salmeterol (50mcg bd) compared with salbutamol (200mcg prn) in children with asthma. European Respiratory Journal 1995;8 Suppl 19:517s.
-
- SMS30013. A multicentre, randomised, double‐blind, crossover study to investigate the efficacy and safety of inhaled salmeterol xinafoate (50µg twice daily from the Diskhaler) compared with placebo (from the Diskhaler) with salbutamol (200µg to use ‘as required’ from the Diskhaler) in children with asthma.. www.ctr.gsk.co.uk.
-
- Zarkovic J, Gotz MH, Holgate ST, Taak NK. Effect of long‐term regular salmeterol treatment in children with moderate asthma. Clinical Drug Investigation 1998;15(3):169‐75.
References to studies excluded from this review
Akpinarli 1999 {published data only}
Arvidsson 1989 {published data only}
-
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Wahlander L, Svedmyr N. Formoterol, a new long acting bronchodilator for inhalation. European Respiratory Journal 1989;2(4):325‐30. - PubMed
Arvidsson 1991 {published data only}
-
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Svedmyr N, Wahlander L. Inhaled formoterol during one year in asthma: a comparison with salbutamol. European Respiratory Journal 1991;4(10):1168‐73. - PubMed
Aziz 1998 {published data only}
-
- Aziz I, Hall IP, McFarlane LC, Lipworth BJ. Beta 2‐adrenceptor regulation and bronchodilator sensitivity after regular treatment with formoterol in subjects with stable asthma. Journal of Allergy & Clinical Immunology 1998;101(3):337‐41. - PubMed
Aziz 1998a {published data only}
-
- Aziz I, Tan KS, Hall IP, Devlin MM, Lipworth BJ. Subsensitivity to bronchoprotection against adenosine monophosphate challenge following regular once‐daily formoterol. European Respiratory Journal 1998;12(3):580‐4. - PubMed
Aziz 1999 {published data only}
-
- Aziz I, Lipworth BJ. A bolus of inhaled budesonide rapidly reverses airway sub‐sensitivity and beta2‐adrenoceptor down‐regulation after regular inhaled formoterol. Chest 1999;115(3):623‐8. - PubMed
Baki 1998 {published data only}
-
- Baki A, Karaguzel G. Short‐term effects of budesonide, nedocromil sodium and salmeterol on bronchial hyperresponsiveness in childhood asthma. Acta Paediatrica Japan 1998;40(3):247‐51. - PubMed
Baumgarten 2002 {published data only}
-
- Baumgarten C, Geldszus R, Behre U, Peslis N, Trautmann M. Initial treatment of symptomatic mild to moderate bronchial asthma with the salmeterol/fluticasone propionate (50/250 microg) combination product (SAS 40023). European Journal of Medical Research 2002;7(1):1‐7. - PubMed
Beach 1993 {published data only}
-
- Beach JR, Young CL, Harkawat R, Gardiner PV, Avery AJ, Coward GA, et al. Effect on airway responsiveness of six weeks treatment with salmeterol. Pulmonary Pharmacology 1993;6(2):155‐7. - PubMed
Becker 1989 {published data only}
-
- Becker AB, Simons F. Formoterol, a new long‐acting selective beta 2‐adrenergic receptor agonist: double‐blind comparison with salbutamol and placebo in children with asthma. Journal of Allergy & Clinical Immunology 1989;84(6):891‐5. - PubMed
Becker 1993 {published data only}
-
- Becker AB, Simons FER. Formoterol, a new beta2 agonist, improves chronic airway hyperresponsiveness in children with asthma. Immunology and Allergy practice 1993;14(7):18‐9.
Bhagat 1995 {published data only}
-
- Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest 1995;108(5):1235‐9. - PubMed
Bishop 1994 {published data only}
-
- Bishop AL, Chervinsky P, Liddle R, et al. Salmeterol for treatment of reversible obstructive airways disease in children. Journal of Allergy & Clinical Immunology 1994;93:248.
Blake 1992 {published data only}
-
- Blake K, Pearlman D, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma, & Immunology 1999;82(2):205‐11. - PubMed
Boner 1992 {published data only}
-
- Boner A. Salmeterol: long term studies in children. European Respiratory Journal 1992;5:318s.
Booth 1996 {published data only}
Boulet 1998a {published data only}
-
- Boulet L, Cartier A, Milot J, Cote J, Malo JL, Laviolette M. Tolerance to the protective effects of salmeterol on methacholine‐induced bronchoconstriction‐ influence of inhaled corticosteroids. European Respiratory Journal 1998;11(5):1091‐7. - PubMed
Boulet 2001 {published data only}
-
- Boulet LP, Chakir J, Milot J, Boutet M, Laviolette M. Effect of salmeterol on allergen‐induced airway inflammation in mild allergic asthma. Clinical & Experimental Allergy 2001;31(3):430‐7. - PubMed
Boulet Laviol 1997 {published data only}
-
- Boulet LP, Laviolette M, Boucher S, et al. A twelve‐ week comparison of salmeterol and salbutamol in the treatment of mild‐to‐moderate asthma: a Canadian multicentre study. Journal of Allergy & Clinical Immunology 1997;99(1 Pt 1):13‐21. - PubMed
Bowers 1997 {published data only}
-
- Bowers BW, Cox FM, Kalberg C, et al. The impact of salmeterol on asthma specific quality of life in patients reporting nocturnal asthma awakenings due to asthma. Annals of Allergy, Asthma, & Immunology 1997;78:110.
Boyd 1995 {published data only}
-
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy‐ UK Study Group. European Respiratory Journal 1995;8(9):1494‐8. - PubMed
Brambilla 1994 {published data only}
-
- Brambilla C, Chastang C, Georges D, et al. Salmeterol compared with slow release terbutaline in nocturnal asthma: a multi center randomised double blind double dummy sequential clinical trial. Allergy 1994;49(6):421‐6. - PubMed
Brambilla 2003 {published data only}
-
- Brambilla C, Gros V, Bourdeix I. Formoterol 12 microg BID administered via single‐dose dry powder inhaler in adults with asthma suboptimally controlled with salmeterol or on‐demand salbutamol: a multicenter, randomized, open‐label, parallel‐group study. Clinical Therapeutics 2003;25(7):2022‐36. - PubMed
Britton 1992 {published data only}
-
- Britton MC, Earnshaw JS, Palmer JBD. A twelve month comparison of salmeterol with salbutamol in asthmatic patients. European Respiratory Journal 1992;5(9):1062‐7. - PubMed
Byrnes 1996 {published data only}
-
- Byrnes CA, Weber EA, Moorat A, et al. A Comparison of salmeterol 50mcg bd and 100mcg bd with salbutamol 200mcg qds in paediatric asthma. American Journal of Respiratory & Critical Care Medicine 1996;153(4):A408.
Byrnes 2000 {published data only}
Campbell 1999 {published data only}
-
- Campbell LM, Anderson TJ, Parashchak MR, Burke CM, Watson SA, Turbitt ML. A comparison of the efficacy of long‐acting beta 2‐agonists: eformoterol via Turbohaler and salmeterol via pressurized metered dose inhaler or Accuhaler, in mild to moderate asthmatics. Respiratory Medicine 1999;93(7):236‐44. - PubMed
Campbell 2000 {published data only}
-
- Campbell LM, Berggren F, Emmas C. The cost effectiveness of eformoterol via Turbohaler(TM) and salmeterol via pressurised metered dose inhaler and metered dose powder inhaler in mild to moderate asthma. Journal of Medical Economics 2000;3:49‐60.
Carlsen 1995 {published data only}
-
- Carlsen K, Roksund O, Olsholt K, Nja F, Leegaard J, Bratten G. Overnight protection by inhaled salmeterol on exercise‐induced asthma in children. European Respiratory Journal 1995;8(11):1852‐5. - PubMed
Castle 1993 {published data only}
Charpin 1992 {published data only}
-
- Charpin D, Vervloet D. Quality of life in moderate chronic asthma: salmeterol versus current treatments. Allergy 1992;47(12 Suppl):230.
Chuchalin 2002 {published data only}
-
- Chuchalin A, Svensson K, Stahl E, Ovcharenko SI, Goriachkina LA, Sidorenko IV, et al. A health‐related quality‐of‐life comparison of formoterol (Oxis) Turbuhaler plus budesonide (Pulmicort) Turbuhaler with budesonide Turbuhaler alone and noncorticosteroid treatment in asthma: a randomized clinical study in Russia. Respiration 2002;69(5):427‐33. - PubMed
-
- Chuchalin AG, Ovcharenko SI, Goriachkina LA, Sidorenko IV, Tsoi AN. The safety and efficacy of formoterol (Oxis) turbuhaler plus budesonide (Pulmicort) turbuhaler in mild to moderate asthma: a comparison with budesonide Turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002;56(1):15‐20. - PubMed
Clark 1993 {published data only}
-
- Clark CE, Ferguson AD, Siddorn J. Respiratory arrests in young asthmatics on salmeterol. Respiratory Medicine 1993;87(3):227‐8. - PubMed
Clauzel 1998 {published data only}
-
- Clauzel AM, Molimard M, Gros V, Lepere E, Febvre N, Michel FB. Use of formoterol dry powder administered for three months via a single dose inhaler in 1,380 asthmatic patients. Journal of Investigational Allergology & Clinical Immunology 1998;8(5):265‐70. - PubMed
Cockcroft 1997 {published data only}
-
- Cockcroft DW, Swystun VA, Bhagat R. Salmeterol and airway response to allergen. Canadian Respiratory Journal 1997;4:37‐40.
Cox 1996 {published data only}
-
- Cox F, Goodwin B, Wenzel S, Rickard K, Kalberg C, Emmett A. Asthma specific quality of life in patients treated with salmeterol or albuterol. Journal of Allergy & Clinical Immunology 1996;97:256.
D'Urzo 2001 {published data only}
-
- D'Urzo AD, Chapman KR, Cartier A, Hargreave FE, Fitzgerald M, Tesarowski D. Effectiveness and safety of salmeterol in non‐specialist practice settings. Chest 2001;119(3):714‐9. - PubMed
D5125C00344 {unpublished data only}
-
- D5125C00344. A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised, parallel group, phase III study to investigate the efficacy and safety of formoterol HFA pMDI compared with placebo and Oxis Turbuhaler in subjects with asthma. www.astrazeneca.com 2005.
De Carli 1995 {published data only}
-
- Carli G, Arpinelli F, Irvine SH, Bamfi F, Ravinetto R, Recchia G. Change of quality of life induced by salmeterol and salbutamol in adult asthmatic patients. Therapie 1995;50 Suppl:N118.
De Oliveira 1998 {published data only}
-
- Oliveira M, Jardim J, Faresin S, Lucas S, Nery L. Efficacy of and tolerance to salmeterol compared to salbutamol in patients with bronchial asthma. Revista da Associacao Medica Brasileira 1998;44(3):169‐75. - PubMed
Derom 1992 {published data only}
-
- Derom EY, Pauwels RA, Straten MEF. The effect of inhaled salmeterol on methacholine responsiveness in subjects with asthma up to 12 hours. Journal of Allergy & Clinical Immunology 1992;89(4):811‐5. - PubMed
Di Lorenzo 1995 {published data only}
-
- Di‐Lorenzo G, Morici G, Norrito F, Mansueto P, Melluso M, Purello‐D'Ambrosio F, et al. Comparison of the effects of salmeterol and salbutamol on clinical activity and eosinophil cationic protein serum levels during the pollen season in atopic asthmatics. Clinical & Experimental Allergy 1995;25(10):951‐6. - PubMed
Droszcz 1999 {published data only}
-
- Droszcz P, Droszcz W. Results of 30 days and 12 months treatment with salmeterol in patients with asthma and COPD. Polski Merkuriusz Lekarski 1999;6:266‐7. - PubMed
Faurschou 1992 {published data only}
-
- Faurschou P. Salmeterol and Salbutamol: long term efficacy and safety. European Respiratory Journal 1992;5:317‐8.
Faurschou 1994a {published data only}
-
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;10(827‐32). - PubMed
Faurschou 1996 {published data only}
-
- Faurschou P, Steffensen I, Jacques L. Effect of addition of inhaled salmeterol to the treatment of moderate‐to‐severe asthmatics uncontrolled on high‐dose inhaled steroids. European Respiratory Journal 1996;9(9):1885‐90. - PubMed
Faurschou 1997 {published data only}
-
- Faurschou P, Dahl R, Jeffery P, et al. Comparison of the anti‐inflammatory effects of fluticasone and salmeterol in asthma‐ A placebo controlled, DB CO with bronchoscopy, bronchial methacholine provocation and lavage [abstract]. European Respiratory Journal 1997;10:243s.
FitzGerald 1999 {published data only}
-
- FitzGerald J, Chapman K, Della Cioppa G, Stubbing D, Fairbarn M, Till M, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. Journal of Allergy & Clinical Immunology 1999;103(31):427‐35. - PubMed
Fitzpatrick 1990 {published data only}
Fuglsang 1998 {published data only}
-
- Fuglsang G, Vikre‐Jorgensen J, Agertoft L, Pedersen S. Effect of salmeterol treatment on nitric oxide level in exhaled air and dose‐response to terbutaline in children with mild asthma. Pediatric Pulmonology 1998;25(5):314‐21. - PubMed
Fuller 1993 {published data only}
-
- Fuller R, Castle W, Palmer J, Hull J. Bronchodilator treatment in asthma. British Medical Journal 1993;306(6892):1611.
Gardiner 1994 {published data only}
-
- Gardiner PV, Ward C, Booth H, et al. Effect of eight weeks treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. American Journal of Respiratory & Critical Care Medicine 1994;150(4):1006‐11. - PubMed
Giannini 1995 {published data only}
-
- Giannini D, Carletti A, Dente FL, et al. Tolerance to salbutamol in allergen induced bronchoconstriction. American Journal of Respiratory & Critical Care Medicine 1995;151:A39.
Giannini 1999 {published data only}
-
- Giannini D, Bacci E, Dente FL, Franco A, Vagagginin B, Testi R, et al. Inhaled beclomethasone dipropionate reverts tolerance to the protective effect of salmeterol on allergen challenge. Chest 1999;115(3):629‐34. - PubMed
Gong 1996 {published data only}
-
- Gong H, Linn WS, Shamou DA, et al. Effect of inhaled salmeterol on sulphur dioxide induced bronchoconstriction in asthmatic subjects. Chest 1996;110(5):1229‐35. - PubMed
Goodwin 1996 {published data only}
-
- Goodwin R, Cox F, Lumry W, et al. The impact of salmeterol versus albuterol on disease specific quality of life in mild to moderate asthmatics. Journal of Allergy & Clinical Immunology 1996;97(3):256.
Green 1002 {published data only}
Greening 1994 {published data only}
-
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344(8917):219‐24. - PubMed
Grove 1995 {published data only}
-
- Grove A, Lipworth BJ. Bronchodilator sub‐sensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet 1995;346(8969):201‐6. - PubMed
Grove 1996 {published data only}
Hacki 1993 {published data only}
-
- Hacki M, Ritter A, Medici T. Formoterol: three years therapy in asthma. European Respiratory Journal 1993;6 Suppl 17:529s.
Hekking 1990 {published data only}
-
- Hekking P, Maesen F, Greefhorst A, et al. Long term efficacy of formoterol compared with salbutamol. Lung 1990;168 Suppl:76‐82. - PubMed
Hermansson 1995 {published data only}
-
- Hermansson B, Jenkins R. A 4‐week comparison of salmeterol and terbutaline in adult asthma. Allergy 1995;50(7):551‐8. - PubMed
Jartti 1998 {published data only}
-
- Jartti TT, Kaila TJ, Tahvanainen KU, Kuusela TA, Vanto TT, Valimaki IA. Altered cardiovascular autonomic regulation after salmeterol treatment in asthmatic children. Clinical Physiology 1998;18(4):345‐53. - PubMed
Jenkins 1991 {published data only}
-
- Jenkins M, Hilton C, Kock J, Palmer J. Exacerbations of asthma in patients on salmeterol. Lancet 1991;337(8746):913‐4. - PubMed
Kalra 1996 {published data only}
-
- Kalra S, Swystun V, Bhagat R, Cockcroft DW. Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest 1996;109(4):953‐6. - PubMed
Kavuru 2000b {published data only}
-
- Kavuru M, Melamed J, Gross G, LaForce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma ‐ a randomized, double‐blind, placebo‐controlled trial. Journal of Allergy & Clinical Immunology 2000;105(6):1108‐16. - PubMed
Kemp 1993 {published data only}
-
- Kemp J, Bukstein D, Busse W. Effective salmeterol, salbutamol and placebo in the prevention of exercise induced bronchospasm. Chest 1993;104 Suppl:10s.
Kemp 1994 {published data only}
-
- Kemp JP, Dockhorn RJ, Busse WW, et al. Prolonged effect of inhaled salmeterol against exercise induced bronchoconstriction after chronic dosing with salmeterol. American Journal of Respiratory & Critical Care Medicine 1994;150(6 Pt 1):1612‐5. - PubMed
Kemp 1998b {published data only}
-
- Kemp J, Cook D, Incaudo G, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids‐ Salmeterol Quality of Life Study Group. Journal of Allergy & Clinical Immunology 1998;101(2 Pt 1):188‐95. - PubMed
Kesten 1991 {published data only}
-
- Kesten S, Chapman KR, Broder I, et al. A three month comparison of inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. American Review of Respiratory Disease 1991;144(3 Pt 1):622‐5. - PubMed
Kesten 1992 {published data only}
-
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. Sustained improvement in asthma with long‐term use of formoterol fumarate. Annals of Allergy 1992;69(5):415‐20. - PubMed
Kozlik‐Feldmann 1996 {published data only}
-
- Kozlik‐Feldmann R, Berg A, Berdel D, Reinhardt D. Long‐term effects of formoterol and salbutamol on bronchial hyperreactivity and beta‐adrenoceptor density on lymphocytes in children with bronchial asthma. European Journal of Medical Research 1996;1(10):465‐70. - PubMed
Lai 1995 {published data only}
-
- Lai C, Chan C, Ho S, Hui A, Lai K. Inhaled salmeterol and albuterol in asthmatic patients receiving high‐dose inhaled corticosteroids. Chest 1995;108(1):36‐40. - PubMed
Langley 1998 {published data only}
-
- Langley SJ, Masterson CM, Batty EP, Woodcock A. Bronchodilator response to salbutamol after chronic dosing with salmeterol or placebo. European Respiratory Journal 1998;11(5):1081‐5. - PubMed
Langton Hewer 1995 {published data only}
-
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrim's progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89(6):435‐40. - PubMed
Lenney 1995 {published data only}
-
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins M. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154(12):983‐90. - PubMed
Li 1999 {published data only}
-
- Li X, Bamford T, Ward C, et al. Influence of salmeterol on eosinophil inflammation in bronchial biopsies from asthmatics on inhaled steroid. European Respiratory Journal 1998;12 Suppl 28:155s.
-
- Li X, Ward C, Thien F, Bish R, Bamford T, Bao X, et al. An antiinflammatory effect of salmeterol, a long‐acting beta2 agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(5 Pt 1):1493‐9. - PubMed
Lipworth 1998 {published data only}
-
- Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. American Journal of Medicine 1998;104(5):431‐8. - PubMed
Lipworth 1999 {published data only}
-
- Lipworth BJ, Aziz I. A high dose of albuterol does not overcome bronchoprotective sub‐sensitivity in asthmatic subjects deceiving regular salmeterol or formoterol. Journal of Allergy & Clinical Immunology 1999;103(1 Pt 1):88‐92. - PubMed
Lipworth 2000a {published data only}
-
- Lipworth B, Aziz I. Bronchodilator response to albuterol after regular formoterol and effects of acute corticosteroid administration. Chest 2000;117(1):156‐62. - PubMed
Lipworth 2000b {published data only}
-
- Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous Glycine‐16 beta2 adrenoceptor polymorphism. Chest 2000;118(2):321‐8. - PubMed
Lotvall 1992 {published data only}
-
- Lotvall J, Lunde H, Ullman A, Tornquist H, Svedmyr N. Twelve months, treatment with inhaled salmeterol in asthmatic patients. effects on b2receptor function and inflammatory cells. Allergy 1992;47(5):477‐83. - PubMed
Lundback 1993 {published data only}
Malo 1992 {published data only}
-
- Malo J‐L, Ghezzo H, Trudeau C. Salmeterol a new inhaled beta2 agonist, has a longer blocking effect than albuterol on hyperventilation induced bronchoconstriction. Journal of Allergy & Clinical Immunology 1992;89(2):567‐74. - PubMed
Mann 1996 {published data only}
-
- Mann RD, Kubota K, Pearce G, et al. Salmeterol: a study by prescription event monitoring in a UK cohort of 15,407 patients. Journal of Clinical Epidemiology 1996;49(2):247‐50. - PubMed
Martin 1999 {published data only}
-
- Martin R, Kraft M, Beaucher W, et al. Comparative study of extended release albuterol sulfate and long‐acting inhaled salmeterol xinafoate in the treatment of nocturnal asthma. Annals of Allergy, Asthma, & Immunology 1999;83(2):121‐6. - PubMed
McIvor 1998 {published data only}
-
- Mcivor RA, Pizzichini E, Turner MO, et al. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1998;158(3):924‐30. - PubMed
Meijer 1995 {published data only}
-
- Meijer GG, Postma DS, Mulder PGH, et al. Long term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 1995;152(6 Pt 1):1887‐92. - PubMed
Midgren 1992 {published data only}
-
- Midgren B, Melander B, Persson G. Formoterol, a new long acting beta2 agonist, inhaled twice daily in stable asthmatic subjects. Chest 1992;101(4):1019‐22. - PubMed
Molimard 2001 {published data only}
-
- Molimard M, Bourcereau V, LeGros V, Bourdeix I, Leynadier F, Duroux P. Comparison between formoterol 12mcg BID and on‐demand salbutamol in moderate persistent asthma. Respiratory Medicine 2001;95(1):64‐70. - PubMed
Nelson 1999a {published data only}
-
- Nelson H, Berkowitz R, Tinkelman D, Emmett A, Rickard K, Yancey S. Lack of sub‐sensitivity to albuterol after treatment with salmeterol in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(5 Pt 1):1556‐61. - PubMed
-
- SLGA5020. A single‐center, randomized, double‐blind, cross‐over clinical trial to examine sub‐sensitivity in adult subjects with asthma receiving salmeterol xinafoate 42mcg BID and placebo BID. www.ctr.gsk.co.uk.
Newnham 1993 {published data only}
-
- Newnham DM, Ingram CG, Earnshaw J, et al. Salmeterol provides prolonged protection against exercise induced bronchoconstriction in a majority of subjects with mild stable asthma. Respiratory Medicine 1993;87(6):439‐44. - PubMed
Nichol 1990 {published data only}
-
- Nichol G, Nix A, Barnes P, Chung K. Prolonged attenuation of BHR to methacholine by long acting b2adrenoceptor agonist formoterol. American Review of Respiratory Disease 1990;141:A 210.
Nielsen 1999 {published data only}
-
- Nielsen L, Pedersen B, Faurschou P, Madsen F, Wilcke J, Dahl R. Salmeterol reduces the need for inhaled corticosteroid in steroid‐dependent asthmatics. Respiratory Medicine 1999;93(12):863‐8. - PubMed
Nightingale 2002 {published data only}
-
- Nightingale JA, Rogers DF, Barnes PJ. Comparison of the effects of salmeterol and formoterol in patients with severe asthma. Chest 2002;121(5):1401‐6. - PubMed
Nix 1990 {published data only}
Norhaya 1999 {published data only}
-
- Norhaya M, Yap T, Zainudin B. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Respirology 1999;4(1):77‐81. - PubMed
Nowak 1992 {published data only}
-
- Nowak D, Jorres R, Rabe KF, et al. Salmeterol protects against hyperventilation induced bronchoconstriction over 12 hours. European Journal of Clinical Pharmacology 1992;43(6):591‐5. - PubMed
O'Byrne 2001 {published data only}
-
- O'Byrne PM, Barnes P, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. American Journal of Respiratory & Critical Care Medicine 2001;164(8 Pt 1):1392‐7. - PubMed
Palmer 1992 {published data only}
-
- Palmer JBD, Stuart AM, Shepherd GL, Viskum K. Inhaled salmeterol in the treatment of patients with moderate to severe ROAD‐ a 3 month comparison of the efficacy and safety of bd salmeterol (100mcg) and salmeterol (50mcg). Respiratory Medicine 1992;86(5):409‐17. - PubMed
Pauwels 1997 {published data only}
-
- Andersson F, Stahl E, Barnes P, Lofdahl C, O'Byrne P, Pauwels R, et al. Adding formoterol to budesonide in moderate asthma‐health economic results from the FACET study. Respiratory Medicine 2001;95(6):505‐12. - PubMed
-
- Juniper E, Svensson K, O'Byrne P, Barnes P, Bauer C, Lofdahl C‐G, et al. Asthma quality of life during 1 year of treatment with budesonide with or without formoterol. European Respiratory Journal 1999;14(5):1038‐43. - PubMed
-
- Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine 1997;337(20):1405‐11. - PubMed
-
- Tattersfield A, Postma D, Barnes P, Svensson K, Bauer C, O'Byrne P, et al. Exacerbations of asthma‐a descriptive study of 425 severe exacerbations‐ The FACET International Study Group. American Journal of Respiratory & Critical Care Medicine 1999;160(2):594‐9. - PubMed
Pearlman 1999 {published data only}
-
- Pearlman D, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: A study comparing monotherapy and combination therapy in asthma. Annals of Allergy 1999;82(3):257‐65. - PubMed
Pederson 1993 {published data only}
-
- Pedersen B, Dahl R, Larsen BB, et al. The effect of salmeterol on the early and late phase reaction to bronchial allergen and post challenge variation in BHR, blood eosinophils, serum ECP and serum eosinophil protein X. Allergy 1993;48(5):377‐82. - PubMed
Price 2002 {published data only}
Quebe‐Fehling 1996 {published data only}
-
- Quebe‐Fehling E, Brambilla R, Bromly CL, Fishwick K, Walters EH, Hendrick D. The duration of action of inhaled formoterol dry powder. British Journal of Clinical Practice 1996;50(8):446‐9. - PubMed
Rabe 1993 {published data only}
-
- Rabe KF, Jorres R, Nowak D, et al. Comparison of the effects of salmeterol and formoterol on airway tone and responsiveness over 24 hours in bronchial asthma. American Review of Respiratory Disease 1993;147(6 Pt 1):1436‐41. - PubMed
Ramsdale 1991 {published data only}
-
- Ramsdale E, Otis J, Kline P, Gontovnick L, Hargreave F, O'Byrne P. Prolonged protection against methacholine induced bronchoconstriction by the inhaled b2 agonist formoterol. American Review of Respiratory Disease 1991;143(5 Pt 1):998‐1001. - PubMed
Rhee 1997 {published data only}
-
- Rhee YK. A comparison of salmeterol with salbutamol inhalation in treatment of mild to moderate asthma. Tuberculosis and Respiratory Diseases 1997;44(4):815‐21.
Ringbaek 1996 {published data only}
-
- Ringbaek TJ, Soes‐Petersen U, Christensen M, Iversen ET, Rasmussen FV. Salmeterol compared with salbutamol controlled release in patients with moderate bronchial asthma. Ugeskrift for Laeger 1996;158(27):3940‐3. - PubMed
Rosenhall 1990 {published data only}
-
- Rosenhall L, Sandstrom T, Wallin A. Formoterol, a long acting inhaled b2 agonist, twice daily for 1 year in asthmatic patients. American Review of Respiratory Disease 1990;141:A 210.
Russell 1995 {published data only}
-
- Russell G, Williams DAJ, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Annals of Allergy, Asthma and Immunology 1995;75(5):423‐8. - PubMed
Ruttenvan Molken1995 {published data only}
-
- Rutten‐van C M, Custers F, van‐Doorslater EKA, et al. Comparison of performance of four instruments in evaluating the effects of salmeterol on asthma quality of life. European Respiratory Journal 1995;8(6):888‐98. - PubMed
Schaaning 1996 {published data only}
-
- Schaanning J, Vilsvik J, Henriksen AH. Efficacy and duration of salmeterol in protecting against exercise induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 1996;76(1):57‐60. - PubMed
Scherner 2004 {published data only}
-
- Schermer TR, Hoff WJ, Greefhorst AP, Creemers JP, Sips AP, Westbroek J, et al. Profiles of measured and perceived bronchodilation. A placebo‐controlled cross‐over trial comparing formoterol and salmeterol in moderate persistent asthma. Pulmonary Pharmacology & Therapeutics 2004;17(4):205‐12. - PubMed
Self 1998 {published data only}
-
- Self T, Rumbak MJ, Kelso T, Eberle L, Abou‐Shala N, Learned CC, et al. Does salmeterol facilitate 'step‐down' therapy in patients with asthma receiving moderate to high doses of inhaled corticosteroids?. Current Therapeutic Research, Clinical & Experimental 1998;59:803‐11.
Shapiro 2000a {published data only}
-
- Shapiro G, Lumry W, Wolfe J, Given J, White M, Woodring A, et al. Combined salmeterol 50 mug and fluticasone propionate 250 in the diskus device for the treatment of asthma. American Journal of Respiratory & Critical Care Medicine 2000;161(2 Pt 1):527‐34. - PubMed
Shepherd 1991 {published data only}
-
- Shepherd G, Jenkins W, Alexander J. Asthma exacerbations in patients taking regular salmeterol, or salbutamol for symptoms. Lancet 1991;337(8754):1424. - PubMed
Sichletidis 1993 {published data only}
-
- Sichletidis L, Daskalopoulou E, Kyriakis G, et al. Comparative efficacy of salbutamol and salmeterol in exercise induced asthma. Journal of International Medical Research 1993;21(2):81‐8. - PubMed
Siergiejko 1998 {published data only}
-
- Siergiejko Z, Zietkowsk1 Z, Rogalewska A, Chyrek‐Borowska S. Clinical evaluation of 12 week salmeterol therapy in allergic asthma patients. Pneumonologia i Alergologia Polska 1998;66(9‐10):450‐5. - PubMed
Simons 1992 {published data only}
-
- Simons F, Soni N, Watson W, Becker A. Bronchodilator and bronchoprotective effects of salmeterol in young patients with asthma. Journal of Allergy & Clinical Immunology 1992;90(5):840‐6. - PubMed
Simons 1997b {published data only}
-
- Simons FER, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise‐induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics. Pediatrics 1997;99(5):655‐9. - PubMed
SLGA2016 {unpublished data only}
-
- SLGA2016. www.fda.gov 2001.
SLGB4011R {unpublished data only}
-
- SLGB4011R. www.ctr.gsk.co.uk 2005.
SMO30003 {unpublished data only}
-
- SMO30003. A single‐centre, randomised, placebo‐controlled, double‐blind, 3‐way crossover proof‐of‐concept study to evaluate the effect of reduced fine particle mass (FPM) from the salmeterol 50mg GR106642X metered dose inhaler compared with salmeterol 50mg P11/12 metered dose inhaler on the peak bronchodilatory effect and bronchoprotection against methacholine‐induced bronchoconstriction. www.ctr.gsk.co.uk 2005.
SMS30035 {published data only}
-
- SMS30035. A double‐blind, three‐way crossover study comparing GR33343G (Salmeterol) 25µg bd, salmeterol 50µg bd and placebo in the treatment of childhood asthma.. www.ctr.gsk.co.uk 2006.
Smyth 1993 {published data only}
Sprogoe‐Jakobsen1992 {published data only}
-
- Sprogoe‐Jakobsen U, Viktrup L, Davidsen O, Viskum K. Bronchial asthma treated with long‐acting beta 2 agonist. Comparison between formoterol (12 mu/g) inhaled twice daily and salbutamol (200 mu/g) inhaled 4 times daily. Ugeskr‐Laeger 1992;154(3):325‐8. - PubMed
Staehr 1995 {published data only}
-
- Staehr P, Vestbo I. Salmeterol improves control in asthmatic patients treated in general practice. A comparative study of salmeterol and salbutamol in patients with mild to moderate asthma. Ugeskrift for Laeger 1995;157(1):36‐40. - PubMed
Steffensen 1996 {published data only}
-
- Steffensen I, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskrift for Laeger 1996;158(49):7092‐6. - PubMed
Tan 1997 {published data only}
-
- Tan KS, Grove A, McLean A, Gnosspelius Y, Hall IP, Lipworth BJ. Systemic corticosteroid rapidly reverses bronchodilator sub‐sensitivity induced by formoterol in asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1997;156(1):28‐35. - PubMed
-
- Tan KS, Hall IP, Dewar JC, Dow E, Lipworth BJ. Association between beta2‐adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet 1997;350(9083):995‐9. - PubMed
Tattersfield 2001 {published data only}
-
- Tattersfield A, Lofdahl C‐G, Postma D, Eivindson A, Schreurs A, Rasidikis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: as randomised trial. The Lancet 2001;357(9252):257‐61. - PubMed
Taylor 1992 {published data only}
-
- Taylor IK, O'Shaugnessy KM, Choudry NB, et al. A comparative study in atopic subjects with asthma of the effects of salmeterol and salbutamol on allergen induced bronchoconstriction, increase in AR ,increase in urinary leukotriene E4 excretion. Journal of Allergy & Clinical Immunology 1992;89(2):575‐83. - PubMed
Taylor Jensen 1997 {published data only}
-
- Taylor DA, Jensen MW, Aikman SL, Harris JG, Barnes PJ, O'Connor BJ. Comparison of salmeterol and albuterol‐induced bronchoprotection against adenosine monophosphate and histamine in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156(6):1731‐7. - PubMed
Thomson 1998 {published data only}
-
- Thomson N, Angus R, Quebe‐Fehling E, Brambilla R. Efficacy and tolerability of formoterol in elderly patients with reversible obstructive airways disease. Respiratory Medicine 1998;92(3):562‐7. - PubMed
Totterman 1998 {published data only}
-
- Totterman KJ, Huhti L, Sutinen E, Backman R, Pietinalho A, Falck M, et al. Tolerability to high doses of formoterol and terbutaline via Turbuhaler(TM) for 3 days in stable asthmatic patients. European Respiratory Journal 1998;12(3):573‐9. - PubMed
Turner 1998 {published data only}
-
- Turner M, Johnston P, Pizzichini E, et al. Anti‐inflammatory effects of salmeterol compared with beclomethasone in eosinophilic mild exacerbations of asthma: a randomized, placebo controlled trial. Canadian Respiratory Journal 1998;5(4):261‐8. - PubMed
Twentyman 1990 {published data only}
-
- Twentyman OP, Finnerty JP, Harris A, et al. Protection against allergen induced asthma by salmeterol. Lancet 1990;336(8727):1338‐42. - PubMed
Ullman 1988 {published data only}
Van der Molen 1996 {published data only}
-
- Molen T, Postma DS, Schreurs AJM, Bosveld HEP, Sears MR, Jong B. Discriminative aspects of two generic and two asthma‐specific instruments: relation with symptoms, bronchodilator use and lung function in patients with mild asthma. Quality of Life Research 1997;6(4):353‐61. - PubMed
-
- Molen T, Sears MR, Graaff CS, Postma DS, Jong B. Quality of life during formoterol treatment: comparison between asthma‐specific and generic questionnaires. European Respiratory Journal 1998;12(1):30‐4. - PubMed
van der Woude 2001 {published data only}
Venables 1992 {published data only}
-
- Venables TA. A multicentre study in general practice to compare the efficacy and tolerability of inhaled salmeterol and terbutaline in the treatment of asthma. British Journal of Clinical Research 1992;3:125‐36.
Verberne 1991 {published data only}
-
- Verberne A, Lenney W, Kerribyjn K. A 3 way crossover study comparing twice daily dosing of salmeterol 25mcg and 50mcg with placebo in children with mild to moderate reversible airways disease [abstract]. American Review of Respiratory Disease 1991;143 Suppl 2:20.
Verberne 1996 {published data only}
-
- Verberne AAPH, Hop WCJ, Creyghton FBM, et al. Airway responsiveness after a single dose of salmeterol and during four months of treatment in children with asthma. Journal of Allergy & Clinical Immunology 1996;97(4):938‐46. - PubMed
Verberne 1997 {published data only}
-
- Verberne A, Frost C, Roorda R, Laag H, Kerrebijn K. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1997;156(3 Pt 1):688‐95. - PubMed
Verberne 1998 {published data only}
-
- Verberne AA, Frost C, Duiverman EJ, et al. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma‐The Dutch Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1998;158(1):213‐9. - PubMed
Verberne 2000 {published data only}
-
- Verberne A, Jongste J. Long‐acting beta2‐agonists in childhood asthma: Don't change a winning team (yet). Pediatric Pulmonology 2000;29(3):169‐71. - PubMed
Verini 1998 {published data only}
-
- Verini M, Verrotti A, Greco R, Chiarelli F. Comparison of the bronchodilator effect of inhaled short‐ and long‐acting beta2‐agonists in children with bronchial asthma. A randomised trial. Clinical Drug Investigation 1998;16(1):19‐224. - PubMed
Wallaert 1999 {published data only}
-
- Wallaert B, Brun P, Ostinelli J, Murciano D, Champel F, Blaive B, et al. A comparison of two long acting beta agonists, oral bambuterol and inhaled salmeterol, in the treatment of asthmatics with nocturnal symptoms. Respiratory Medicine 1999;93(1):33‐8. - PubMed
Walters 1992 {published data only}
-
- Walters H. Quality of life in long term studies [abstract]. European Respiratory Journal 1992;5:318s.
Weinstein 1997 {published data only}
-
- Weinstein S, Chervinsky P, Pollard SJ, Bronsky EA, Nathan RA, Prenner B, et al. A one‐week dose‐ranging study of inhaled salmeterol in children with asthma. Journal of Asthma 1997;34(1):43‐52. - PubMed
Wenzel 1998 {published data only}
-
- Wenzel SE, Lumry W, Manning M, Kalberg C, Cox F, Emmett A, et al. Efficacy, safety, and effects on quality of life of salmeterol versus albuterol in patients with mild to moderate persistent asthma. Annals of Allergy 1998;80(6):463‐70. - PubMed
Wilding 1997 {published data only}
Wolfe 1995 {published data only}
-
- Wolfe J, LaForce C, Chervinsky P, Galant S, Lumry W, Noonan M, et al. Inhaled salmeterol powder compared with albuterol aerosol given regularly or as needed for asthma. Annals of Allergy, Asthma and Immunology 1995;74:52.
Wong 1997 {published data only}
-
- Wong AG, O'Shaughnessy AD, Walker CM, Sears MR. Effects of long‐acting and short‐acting beta‐agonists on methacholine dose‐response curves in asthmatics. European Respiratory Journal 1997;10(2):330‐6. - PubMed
Woolcock 1996 {published data only}
-
- Woolcock A, Lundback B, Ringdal N, et al. Comparison of the addition of salmeterol to inhaled steroids with the doubling of the dose of inhaled steroids. American Journal of Respiratory & Critical Care Medicine 1996;153(5):1481‐8. - PubMed
Yates 1995 {published data only}
-
- Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma. Effect on bronchial responsiveness during and after treatment. American Journal of Respiratory & Critical Care Medicine 1995;152(4 Pt 1):1170‐4. - PubMed
Yates 1997 {published data only}
-
- Yates DH, Worsdell M, Barnes PJ. Effect of regular salmeterol treatment on albuterol‐induced bronchoprotection in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156(3):988‐91. - PubMed
Zellweger 1994 {published data only}
-
- Zellweger JP, Plenninger M, Ruff P. 24 hour protective effect of salmeterol 50mg v formoterol 24 mg against methacholine induced bronchoconstriction in mild to moderate asthmatic patients: a randomised double blind crossover single dose trial. European Respiratory Journal 1994;7 Suppl 18:422s.
Zimmerman 2004 {published data only}
-
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004;37(2):122‐7. - PubMed
Additional references
AAACI 1993
-
- American Academy of Allergy and Clinical Immunology. Inhaled beta2 agonist in asthma. Journal of Allergy & Clinical Immunology 1993;91(6):1234‐7. - PubMed
Abramson 2001
-
- Abramson M, Bailey M, Couper F, Driver J, Drummer O, Forbes A, et al. Are asthma medications and management related to deaths from asthma?. American Journal of Respiratory & Critical Care Medicine 2001;163(1):12‐8. - PubMed
Adcock 1996
-
- Adcock I, Stevens D, Barnes P. Interactions of glucocorticoids and beta2 agonists. European Respiratory Journal 1996;9(1):160‐9. - PubMed
Adkins 1997
-
- Adkins JC, McTavish D. Salmeterol. A review of its pharmacological properties and clinical efficacy in the management of children with asthma. Drugs 1997;54(2):331‐54. - PubMed
Altman 2003
Bartow 1998
-
- Bartow RA, Brogen RN. Formoterol. An update of its pharmacological properties and therapeutic efficacy in the management of asthma. Drugs 1998;55(2):303‐22. - PubMed
Beasley 1999
-
- Beasley R, Pearce N, Crane J, Burgess C. Beta‐agonists: What is the evidence that their use increases the risk of asthma morbidity and mortality?. Journal of Allergy & Clinical Immunology 1999;104(2 Pt 2):18S‐30S. - PubMed
Bisgaard 2000
-
- Bisgaard H. Long‐acting beta(2)‐agonists in management of childhood asthma: A critical review of the literature. Pediatric Pulmonology 2000;29(3):221‐34. - PubMed
Boulet 1994
-
- Boulet LP. Long versus short acting beta agonists. Drugs 1994;47(2):207‐22. - PubMed
Boyer 1992
-
- Boyer G, Aaronson G, Meltzer E, LaForce C, Grossman J, Yancey S. Enhancement of a general quality of life scale: validation of a sleep scale [abstract]. Journal of Allergy & Clinical Immunology 1992;89:186.
BTS 1993
BTS 1995
-
- British Thoracic Society et al. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52 Suppl 1:S1‐S20.
BTS summary 1993
Campbell 1976
-
- Campbell A. Mortality from asthma and bronchodilator aerosols. Medical Journal of Australia 1976;1(12):386‐91. - PubMed
Cates 2012
Cates 2014
Christie 1993
-
- Christie M, French D, Sowden A, West A. The development of child centred, disease specific questionnaires for living with asthma. Psychosomatic Medicine 1993;55(6):541‐8. - PubMed
Cockcroft 1993
-
- Cockcroft DW, McParland CP, Britto SA. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993;342(8875):833‐7. - PubMed
Cockcroft 1996
Crane 1989
-
- Crane J, Pearce N, Flatt A, et al. Prescribed fenoterol and death from asthma in New Zealand 1981‐1983. A case control study. Lancet 1989;1(8644):917‐22. - PubMed
D'Urzo 1997
Devoy 1995
-
- Skorodin MS. Asthma mortality and beta agonists. Chest 1995;108(6):1768. - PubMed
Devoy Fuller 1995
-
- Devoy MA, Fuller RW, Palmer JB. Are there any detrimental effects of the use of inhaled long‐acting beta 2‐agonists in the treatment of asthma?. Chest 1995;107(4):1116‐24. - PubMed
Faurschou 1994
-
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;49(10):827‐32. - PubMed
French 1994
-
- French DJ, Christie MJ, Sowden AJ. The reproducibility of the Childhood Asthma Questionnaires: measures of quality of life for children with asthma aged 4‐16 yrs. Quality of Life Research 1994;3(3):215‐24. - PubMed
Fuller 1995
-
- Fuller R. Safety of salmeterol in the treatment of asthma. European Respiratory Review 1995;5(27):133‐7.
Giannini 2000
-
- Giannini D, Franco A, Bacci E, Dente FL, Taccola M, Vagaggini B, et al. The protective effect of salbutamol inhaled using different devices on methacholine bronchoconstriction. Chest 2000;117(5):1319‐23. - PubMed
GINA 1995
-
- National Heart Lung and Blood Institute/WHO. Global strategy for asthma management and prevention. Bethesda: National Institutes of Health, 1995.
GINA 2000
-
- Bousquet J. Global initiative for asthma (GINA) and its objectives. Clinical & Experimental Allergy 2000;30 Suppl 1:S2‐5. - PubMed
Greenstone 2005
-
- Greenstone IR, Ni Chroinin M, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 4. - PubMed
Hawkins 2006
Higgins 2003
Howarth 2000
-
- Howarth PH, Beckett P, Dahl R. The effect of long‐acting beta2‐agonists on airway inflammation in asthmatic patients. Respiratory Medicine 2000;94 Suppl F:S22‐5. - PubMed
Hyland 1991
-
- Hyland ME. The Living with Asthma Questionnaire. Respiratory Medicine 1991;85 Suppl B:13‐6. - PubMed
Jadad 1996
-
- Jadad A, Moore R, Carroll D, Jenkinson C, Reynolds J, Gavaghan D, et al. Assessing the quality of reports of randomised control trials: is blinding really necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Janson 2001
-
- Janson C, Anto J, Burney P, et al. The European community respiratory health survey: what are the main results so far?. European Respiratory Journal 2001;18(3):598‐611. - PubMed
Johnson 2004
-
- Johnson M. Interactions between corticosteroids and beta2‐agonists in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2004;1(3):200‐6. - PubMed
Juniper 1992
Juniper 1994
-
- Juniper EF, Guyatt GH, Willan A, Griffith L. Determining a minimal important change in a disease‐specific quality of life questionnaire. Journal of Clinical Epidemiology 1994;47(1):81‐7. - PubMed
Kips 2001
-
- Kips J, Pauwels R. Long‐acting inhaled beta(2)‐agonist therapy in asthma. American Journal of Respiratory & Critical Care Medicine 2001;164(6):923‐32. - PubMed
Lai 1989
-
- Lai CKW, Twentyman OP, Holgate ST. The effect of an increase in inhaled allergen dose after rimiterol hydrobromide on the occurrence and magnitude of the late asthmatic response and the associated change in nonspecific bronchial hyperresponsiveness. American Review of Respiratory Disease 1989;140(4):917‐23. - PubMed
Lipworth 1999a
-
- Lipworth BJ, Hall IP, Aziz I, Tan KS, Wheatley A. Beta2‐adrenoceptor polymorphism and bronchoprotective sensitivity with regular short and long‐acting beta2‐agonist therapy. Clinical Science 1999;96(3):253‐9. - PubMed
Lipworth Hall 1999
-
- Lipworth B, Hall I, Tan S, Aziz I, Coutie W. Effects of genetic polymorphism on ex vivo and in vivo function of beta2‐adrenoceptors in asthmatic patients. Chest 1999;115(2):324‐8. - PubMed
Moore 1996
-
- Moore RH, Khan A, DIckey B. Long‐acting inhaled beta2‐agonists in asthma therapy. Chest 1996;113(4):1095‐8. - PubMed
NAC 2002
-
- National Asthma Campaign. Asthma management handbook. Melbourne: National Asthma Council Australia Ltd, 2002.
Ni Chroinin 2004
Ni Chroinin 2005
-
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 4. - PubMed
Page 1993
-
- Page CP. An explanation of the asthma paradox. American Review of Respiratory Disease 1993;147(6 Pt 2):S29‐32. - PubMed
Palmquist 1997
-
- Palmqvist M, Persson G, Lazer L, Rosenborg J, Larsson P, Lotvall J. Inhaled dry‐powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potency. European Respiratory Journal 1997;10(11):2484‐9. - PubMed
Partridge 2000
-
- Partridge M, Fabbri L, Chung K. Delivering effective asthma care‐ how do we implement asthma guidelines?. European Respiratory Journal 2000;15(2):235‐7. - PubMed
Pearce 2000
-
- Pearce N, Sunyer J, Cheng S, et al. Comparison of asthma prevalence in the ISAAC and the ECRHS. ISAAC Steering Committee and the European Community Respiratory Health Survey. International Study of Asthma and Allergies in Childhood. European Journal of Respiratory Medicine 2000;16(3):420‐6. - PubMed
Reid 2003
-
- Reid DW, Ward C, Wang N, Zheng L, Bish R, Orsida B, et al. Possible anti‐inflammatory effect of salmeterol against interleukin‐8 and neutrophil activation in asthma in vivo. European Respiratory Journal 2003;21(6):994‐9. - PubMed
Salpeter 2006
-
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Ann Intern Med 2006;144(12):904‐12. - PubMed
Sears 1986
Sears 1998
-
- Sears MR. Asthma treatment: inhaled beta‐agonists. Canadian Respiratory Journal 1998;5 Suppl A:54A‐9A. - PubMed
Selroos 1998
-
- Selroos O. The pharmacologic and clinical properties of Oxis (formoterol) Turbuhaler. Allergy 1998;53(42 Suppl):14‐9. - PubMed
Shale 1994
-
- Shale DJ. Respiratory arrests in young asthmatics on salmeterol. Respiratory Medicine 1994;88(1):75‐6. - PubMed
Shrewsbury 2002
-
- Shrewsbury S, Pyke S, Sears M. MIASMA: asthma exacerbation reduction with salmeterol. Chest 2002;121(3):1002‐4. - PubMed
Sont 1999
-
- Sont JK, Willems LN, Bel EH, Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long‐term treatment. The AMPUL Study Group. American Journal of Respiratory & Critical Care Medicine 1999;159(4 Pt 1):1043‐51. - PubMed
Spitzer 1992
-
- Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, et al. The use of beta‐agonists and the risk of death and near death from asthma. New England Journal of Medicine 1992;326(8):501‐6. - PubMed
Stein 1990
-
- Stein R, Jessup D. Functional status II(R). A measure of child health status. Medical Care 1990;28(11):1041‐55. - PubMed
Sullivan 1996
-
- Sullivan S, Elixhauser A, Buist A, Luce B, Eisenberg J, Weiss K. National Asthma Education and Prevention Program working group report on the cost effectiveness of asthma care. Program working group report on the cost effectiveness of asthma care. American Journal of Respiratory & Critical Care Medicine 1996;154(3 Pt 2):S84‐95. - PubMed
Tattersfield 1993
-
- Tattersfield AE. Clinical pharmacology of long‐acting beta‐receptor agonists. Life Sciences 1993;52(26):2151‐9. - PubMed
Taylor 1997
-
- Taylor D, Jensen M, Aikman S, Harris J, Barnes PJ, O'Connor B. Comparison of salmeterol and albuterol induced bronchoprotection against AMP and histamine in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156(6):1731‐7. - PubMed
Taylor 2000
TSANZguidelines 1989
-
- Woolcock A. Rubinfeld AR. Seale JP. Landau LL. Antic R. Mitchell C, et al. Thoracic society of Australia and New Zealand. Asthma management plan. Medical Journal of Australia 1989;151(11‐12):650‐3. - PubMed
Van der Molen 1997
-
- Molen T, Postma DS, Schreurs AJM, Bosveld HEP, Sears MR, Meyboom de Jong B. Discriminative aspects of two generic and two asthma‐specific instruments: relation with symptoms, bronchodilator use and lung function in patients with mild asthma. Quality of Life Research 1997;6(4):353‐61. - PubMed
van Noord 1996
-
- Noord J, Smeets J, Raaijmakers J, Bommer A, Maesen F. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9(8):1684‐8. - PubMed
Van Schayck 2000
-
- Schayck C. Do long‐acting beta2‐adrenergic agonists deserve a different place in guidelines for the treatment of asthma and COPD?. European Respiratory Journal 2000;15(4):631‐4. - PubMed
Verberne 1995
-
- Verberne AAPH, McCormack EM, Fuller R, et al. An overview of nine clinical trials of salmeterol in an asthmatic population. European Respiratory Journal 1995;8(Suppl 19):156S. - PubMed
Vervloet 1998
-
- Vervloet D, Ekstrom T, Pela R, Duce Gracia F, Kopp C, Silvert BD, et al. A 6‐month comparison between formoterol and salmeterol in patients with reversible obstructive airways disease. Respiratory Medicine 1998;92(6):836‐42. - PubMed
Weinberger 1995
-
- Weinberger M. Salmeterol for the treatment of asthma. Annals of Allergy, Asthma and Immunology 1995;75(3):209‐11. - PubMed
Woods 2001
-
- Woods R, Walters E, Wharton C, Watson N, Abramson M. The rising prevalence of asthma in young Melbourne adults is associated with improvement in treatment. Annals of Allergy, Asthma, & Immunology 2001;87(2):117‐23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

