Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia
- PMID: 17253515
- PMCID: PMC6464930
- DOI: 10.1002/14651858.CD004597.pub2
Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia
Abstract
Background: Cancer cachexia is a distressing weight loss syndrome commonly seen in advanced cancer patients. It is associated with reduced quality of life and shorter survival time. Eicosapentaenoic acid (EPA) is a long chain polyunsaturated fatty acid found naturally in some fish which has been used to decrease weight loss, promote weight gain and increase survival times in patients affected with cancer cachexia.
Objectives: To evaluate the effectiveness and safety of EPA in relieving symptoms associated with the cachexia syndrome in patients with advanced cancer.
Search strategy: Studies were sought through an extensive search of a range of electronic databases. Hand searching was conducted on selected journals and reference lists as well as contact made with investigators, manufacturers and experts. The most recent electronic search was conducted in February 2005.
Selection criteria: Studies were included in the review if they assessed oral EPA compared with placebo or control in randomised controlled trials of patients with advanced cancer and either a clinical diagnosis of cachexia or self-reported weight loss of 5% or more.
Data collection and analysis: Both methodological quality evaluation of potential trials and data extraction were conducted by two independent review authors.
Main results: Five trials (involving 587 patients) met the inclusion criteria. Three trials compared EPA at different doses with placebo with two outcomes, nutritional status and adverse events comparable across two of the three included trials. In addition, two trials compared different doses of EPA with an active matched control. It was possible to compare the outcomes of weight, quality of life and adverse events across these two trials. There were insufficient data to define the optimal dose of EPA.
Authors' conclusions: There were insufficient data to establish whether oral EPA was better than placebo. Comparisons of EPA combined with a protein energy supplementation versus a protein energy supplementation (without EPA) in the presence of an appetite stimulant (Megestrol Acetate) provided no evidence that EPA improves symptoms associated with the cachexia syndrome often seen in patients with advanced cancer.
Conflict of interest statement
None known
Figures
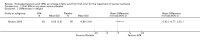
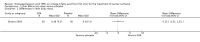


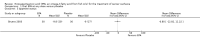

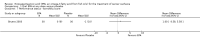





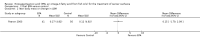

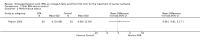


Update of
References
References to studies included in this review
Bruera 2003 {published data only}
-
- Bruera E, Strasser F, Palmer JL, Willey J, Calder K, Amyotte G, Baracos V. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double‐blind, placebo‐controlled study. Journal of Clinical Oncology 2003;21(1):129‐34. - PubMed
Fearon 2003 {published and unpublished data}
Gogos 1998 {published data only}
-
- Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega‐3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy. Cancer 1998;82(2):395‐402. - PubMed
Jatoi 2004 {published data only}
-
- Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer‐associated wasting: a north central cancer treatment group and national cancer institute of Canada collaborative effort. Journal of Clinical Oncology 2004; Vol. 22, issue 12:2469‐76. - PubMed
Zuijdgeest 2000 {published data only}
-
- Zuijdgeest‐van Leeuwen SD, Dagnelie PC, Wattimena JLD, Berg JWO, Gaast A, Swart GR, et al. Eicosapentaenoic acid ethyl ester supplementation in cachectic cancer patients and healthy subjects: effects on lipolysis and lipid oxidation. Clinical Nutrition 2000;19(6):417‐23. - PubMed
References to studies excluded from this review
Atkinson 1998 {published data only}
-
- Atkinson S, Sieffert E, Bihari D. A prospective randomized, double blind, controlled clinical trial of enteral immunonutrition in the critically ill. Crit Care Med 1998;26(7):1164‐72. - PubMed
Barber 1998 {published data only}
-
- Barber MD, Ross JA, McMillan DC, Preston T, Shenkin A, Fearon KCH. A fish oil‐enriched nutritional supplement modulates changes in the acute phase protein response in weight‐losing pancreatic cancer patients. Clinical Nutrition 1998;17(1 Supplement):41. [P.44]
Barber 1999a {published data only}
Barber 1999b {published data only}
-
- Barber MD, Ross JA, Preston T, Shenkin A, Fearon KCH. Fish oil‐enriched nutritional supplement attenuates progression of the acute‐phase response in weight‐losing patients with advanced pancreatic cancer. Journal of Nutrition 1999;129(6):1120‐5. - PubMed
Barber 2000 {published data only}
-
- Barber MD, McMillan DC, Preston T, Ross JA, Fearon KCH. Metabolic response to feeding in weight‐losing pancreatic cancer patients and its modulation by a fish‐oil‐enriched nutritional supplement. Clinical Science 2000;98:389‐99. - PubMed
Barber 2001a {published data only}
-
- Barber MD, Fearon KCH. Tolerance and incorporation of a high‐dose eicosapentaenoic acid diester emulsion by patients with pancreatic cancer cachexia. Lipids 2001;36(4):347‐51. - PubMed
Barber 2001b {published data only}
-
- Barber MD, Fearon KCH, Tisdale MJ, McMillan DC, Ross JA. Effect of a fish oil‐enriched nutritional supplement on metabolic mediators in patients with pancreatic cancer cachexia. Nutrition and cancer 2001;40(2):118‐24. - PubMed
Barber 2004 {published data only}
-
- Barber M, Preston T, McMillan D, Slaters C, Ross J, Fearon KCH. Modulation of the liver export protein synthetic response to feeding by an n‐3 fatty acid enriched nutritional supplement is associated with anabolism in cachectic cancer patients. Clinical Science 2000;106:359‐64. - PubMed
Bauer 2005 {published data only}
-
- Bauer JD, Capra S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy ‐ a pilot study. Support Care Cancer 2005;13:270‐4. - PubMed
Braga 1995 {published data only}
-
- Braga M, Vignali A, Gianotti L, Cestari A, Profili M, Carlo V. Benefits of Early Postoperative Enteral Feeding in Cancer Patients. Infusionsther Transfusionsmed 1995;22:280‐4. - PubMed
Braga 1996a {published data only}
-
- Braga M, Vignali A, Gianotti L, Cestari A, Profili M, Carlo V. Immune and nutritional effects of early enteral nutrition after major abdominal operations. European Journal Surgery 1996;162:105‐12. - PubMed
Braga 1996b {published data only}
-
- Braga M, Gianotti L, Cestari A, Vignali A, Pellegatta F, Dolci A, Carlo V. Gut function and immune and inflammatory responses in patients preoperatively fed with supplemented enteral formulas. Archives of Surgery 1996;131(12):1257‐65. - PubMed
Braga 1999 {published data only}
-
- Braga M, Gianotti L, Radaelli G, Vignali A, Mari G, Gentilini O, Carlo V. Perioperative immunonutrition in patients undergoing cancer surgery. Archives of Surgery 1999;134(4):428‐33. - PubMed
Braga 2002 {published data only}
-
- Braga M, Gianotti L, Vignali A, Carlo V. Preoperative oral arginine and n‐3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery 2002;132(5):805‐14. - PubMed
Braga 2002a {published data only}
-
- Braga M, Gianotti L, Nespoli L, Radaelli G, Carlo V. Nutritional Approach in Malnourished Surgical Patients. Archives of Surgery 2002;137:174‐80. - PubMed
Brosnahan 2003 {published data only}
-
- Brosnahan J. Supplementation with key nutrients reduced postoperative infections and length of hospital stay after gastrointestinal surgery. Evidence‐Based Nursing 2003;6(2):47. - PubMed
Burns 1999 {published data only}
-
- Burns CP, Halabi S, Clamon GH, Hars V, Wagner BA, Hohl RJ, et al. Phase I Clinical Study of fish oil fatty acid capsules for patients with cancer cachexia: cancer and leukemia Group B Study 9473. Clinical Cancer Research 1999;5:3942‐7. - PubMed
Burns 2004 {published data only}
-
- Burns CP, Halabi S, Clamon G, Kaplan E, Hohl RJ, Atkins JN, et al. Phase II Study of High‐Dose fish oil capsules for patients with cancer‐related cachexia. Cancer 2004;101(2):370‐8. - PubMed
Daly 1992 {published data only}
-
- Daly JM, Lieberman MD, Goldfine J, Shou J, Weintraub F, Rosato EF, et al. Enteral nutrition with supplemental arginine, RNA and omega‐3 fatty acids in patients after operation: Immunologic, metabolic and clinical outcome. Surgery 1992;112(1):56‐67. - PubMed
Daly 1995 {published data only}
Davidson 2004 {published data only}
-
- Davidson W, Ash S, Capra S, Bauer J. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clinical Nutrition 2004;23:239‐47. - PubMed
Di Carlo 1999 {published data only}
-
- Carlo V, Gianotti L, Balzano G, Zerbi A, Braga M. Complications of pancreatic surgery and the role of perioperative nutrition. Digestive Surgery 1999;16:320‐6. - PubMed
Falconer 1994 {published data only}
-
- Falconer JS, Fearon KCH, Ross JA, Carter DC. Polyunsaturated fatty acids in the treatment of weight‐losing patients with pancreatic cancer. World Rev Nutritional Diet 1994;76:74‐6. - PubMed
Fearon 2001 {published data only}
-
- Fearon KCH, Meyenfeldt M, Moses AGW, Geenen R, Roy A, Gouma D, et al. An energy and protein dense, high n‐3 fatty acid oral supplement promotes weight gain in cancer cachexia. European Journal of Cancer 2001;37(Supplement 6):90.
Gianotti 1997 {published data only}
-
- Gianotti L, Braga M, Vignali A, Balzano G, Zerbi A, Bisagni P, Carlo V. Effect of route of delivery and formulation of postoperative nutritional support in patients undergoing major operations for malignant neoplasms. Archives of Surgery 1997;132(11):1222‐30. - PubMed
Gianotti 1999 {published data only}
-
- Gianotti L, Braga M, Fortis C, Soldini L, Vignali A, Colombo S, et al. A prospective randomized clinical trial on perioperative feeding with an arginine, omega‐3 fatty acid, and RNA‐enriched enteral diet: effect on host response and nutritional status. Journal of Parenteral and Enteral Nutrition 1999;23(6):314‐20. - PubMed
Gianotti 2002 {published data only}
-
- Gianotti L, Braga M, Nespoli L, Radelli G, Beneduce A, Carlo V. A randomised controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002;122(7):1763‐70. - PubMed
Gramaglia 1999 {published data only}
-
- Gramaglia A, Loi GF, Mongioj V, Baronzio F. Increased survival in brain metastatic patients treated with stereotatic radiotherapy, omega three fatty acids and bioflavonoids. Anticancer Research 1999;19:5583‐6. - PubMed
Heller 2004 {published data only}
-
- Heller AR, Rossel T, Gottschlich B, Tiebel O, Menschikowski M, Litz RJ, et al. Omega‐3 fatty acids improve liver and pancreas function in postoperative cancer patients. International Journal Cancer 2004;111:611‐6. - PubMed
Heslin 1997 {published data only}
Keman 1995 {published data only}
-
- Keman M, Senkal M, Homann HH, Mumme A, Dauphin AK, Baier J, et al. Early postoperative enteral nutrition with arginine‐omega‐3 fatty acids and ribonucleic acid‐supplemented diet versus placebo in cancer patients: An immunologic evaluation of Impact Registered Trademark. Critical Care Medicine 1995;23(4):652‐9. - PubMed
Mantovani 2004 {published data only}
-
- Mantovani G, Madeddu C, Maccio A, Gramignano G, Lusso MR, Massa E, et al. Cancer‐related anorexia.cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Cancer Epidemiology, Biomarkers & Prevention 2004;13(10):1651‐9. - PubMed
McCarter 1998 {published data only}
-
- McCarter MD, Gentilini OD, Gomez ME, Daly JM. Preoperative oral supplement with immunonutrients in cancer patients. Journal of Parenteral and Enteral Nutrition 1998;22(4):206‐11. - PubMed
Moses 2001 {published data only}
-
- Moses AG, Slater C, Barber MD, Fearon KC, Preston T. An experimental nutrition supplement enriched with n‐3 fatty acids and antioxidants is associated with an increased physical activity level in patients with pancreatic cancer cachexia. Clinical Nutrition 2001;20(62 S3):21.
Moses 2002 {published data only}
-
- Moses AG, Slater C, Barber MD, Preston T, Fearon KCH. Physical activity level in patients with pancreatic cancer cachexia. British Journal Surgery 2002;89(Suppl 1):42.
Persson 2005 {published data only}
-
- Persson C, Glimelius B, Ronnelid J, Nygren P. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: a randomized pilot study. Nutrition 2005;21:170‐8. - PubMed
Pratt 2002 {published data only}
Read 2004 {published and unpublished data}
-
- Read JA, Clarke SJ, Volker D. Nutritional and anti‐inflammatory strategies in the treatment of advanced colorectal cancer: a pilot study. Asia Pacific Journal of Clinical Nutrition 2004;13 (Supplement):S93.
Rodrigo 1997 {published data only}
-
- Rodrigo MP, Casanova y JM, Pena G. Influence of the composition of the enteral nutrition on the infection of the critical patient [Influencia de la composicion de la nutricion enteral en la infeccion del paciente critico]. Nutricion Hospitalaria 1997;XII(2):80‐4. - PubMed
Schilling 1995 {published data only}
-
- Schilling J, Vranjes N, Fierz W, Joller H, Gyurech D, Ludwig E, et al. Clinical outcome and immunology of postoperative arginine, w‐3 fatty acids, and nucleotide‐enriched enteral feeding: a randomized prospective comparison with standard enteral and low calorie/low fat IV solutions. Nutrition 1996;12(6):423‐9. - PubMed
Senkal 1995 {published data only}
-
- Senkal M, Kemen M, Homann HH, Eickhoff U, Baier J, Zumtobel V. Modulation of Postoperative Immune Response by Enteral Nutrition with a Diet Enriched with Arginine, RNA, and Omega‐3 Fatty Acids in Patients with Upper Gastrointestinal Cancer. European Journal Surgery 1995;161:115‐22. - PubMed
Senkal 1997 {published data only}
-
- Senkal M, Mumme A, Eickhoff U, Geier B, Spath G, Wulfert D, et al. Early postoperative enteral immunonutrition clinical outcome and cost‐comparison analysis in surgical patients. Critical Care Medicine 1997;25(9):1489‐96. - PubMed
Senkal 1999 {published data only}
-
- Senkal M, Zumtobel V, Bauer KH, Marpe B, Wolfram G, Frei A, et al. Outcome and cost‐effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery. Archives of Surgery 1999;134(12):1309‐16. - PubMed
Swails 1997 {published data only}
-
- Swails WS, Kenler AS, Driscoll DF, DeMichele SJ, Babineau TJ, Utsunamiya T, et al. Effect of a fish oil structured lipid‐based diet on prostaglandin release from mononuclear cells in cancer patients after surgery. Journal of Parenteral and Enteral Nutrition 1997;21(5):266‐74. - PubMed
Synderman 1999 {published data only}
-
- Synderman CH, Kachman K, Molseed L, Wagner R, D'Amico F, Bumpous J, Rueger R. Reduced postoperative infections with an immune‐enhancing nutritional supplement. The Laryngoscope 1999;109:915‐21. - PubMed
Tashiro 1998a {published data only}
-
- Tashiro T, Yamamori H, Takagi K, Hayashi N, Furukawa K, Nakajima N. n‐3 verus n‐6 polyunsaturated fatty acids in critical illness. Nutrition 1998;14(6):551‐3. - PubMed
Tashirom 1998b {published data only}
-
- Tashiro T, Yamamori H, Takagi K, Hayashi N, Furukawa K, Nakajima N. n‐3 versus n‐6 polyunsaturated fatty acids in critical illness. Nutrition 1998;14(6):551‐3. - PubMed
Vignali 1995 {published data only}
-
- Vignali A, Braga M, Gianotti L, Cestari A, Profili M, Carlo V. Impact of an enriched enteral formula on immune function and nutritional status in cancer patients following surgery [Immunonutrizione enterale precoce nel paziente oncologico chirugico: valutazione immunologica e nutrizonale]. Rivista Italiana di Nutrizione Parenterale ed Enterale 1995;13(1):25‐31.
vonMeyenfeldt 2002 {published data only}
-
- vonMeyenfeldt M, Ferguson M, Voss A, Fearon K, Moses A, Geene R, Gouma DJ, Roy A, Giacosa A, Gossom M, Tisdale M. Weight gain is associated with improved quality of life in patients with cancer cachexia consuming an energy and protein dense, high n‐3 fatty acid oral supplement. Proceedings American Society of Clinical Oncology 2002;21:385A.
Wachtler 1995 {published data only}
-
- Wachtler P, Hilger RA, Konig W, Bauer KH, Kemen M, Koller M. Influence of a pre‐operative enteral supplement on functional activities of peripheral leukocytes from patients with major surgery. Clinical Nutrition 1995;14:275‐82. - PubMed
Wigmore 1996 {published data only}
-
- Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, Carter DC, Fearon KCH. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 1996;12(1):S27‐30. - PubMed
Wigmore 1997 {published data only}
-
- Wigmore SJ, Fearon KCH, Maingay JP, Ross JA. Down‐regulation of the acute‐phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin‐6. Clinical Science 1997;92:215‐21. - PubMed
Wigmore 2000 {published data only}
-
- Wigmore SJ, Barber MD, Ross JA, Tisdale MJ, Fearon KCH. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutrition and Cancer 2000;36(2):177‐84. - PubMed
References to ongoing studies
Fearon 2005 {unpublished data only}
-
- A DBPCR Multi‐centre Phase Dose Response study of EPA 95% Diester Capsules in patients with cancer cachexia. Ongoing study 1998.
Additional references
Altman 2001
-
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of Internal Medicine 2001;134:663‐94. - PubMed
Beck 1991
-
- Beck SA, Smith KL, Tisdale MJ. Anticachectic and antitumour effect of Eicosapentaenoic acid and its effect on protein turnover. Cancer Research 1991;51:6089‐91. - PubMed
Belizario 1991
Blackburn 1977
-
- Blackburn GL, Bistrian BR, Main BS, Schlamm HT, Smith MF. Nutritional and metabolic assessment of the hospitalized patient. The Journal of Parenteral and Enteral Nutrition 1977;1:11‐22. - PubMed
Brodie 1998
-
- Brodie D, Moscrip V, Hutcheon R. Body composition measurement: a review of hydrodosensitometry, anthropometry and impedance methods. Nutrition 1998;14:14‐5. - PubMed
DeWys 1980
-
- DeWys WD, Begg C, Lavin PT, Band PR, Bennett JM. Prognostic effect of weight‐loss prior to chemotherapy in cancer patients. American Journal of Medicine 1980;69:491‐7. - PubMed
Giacosa 1994
-
- Giacosa A, Frascio F, Sukkar SG, Roncella S. Food intake and body composition in cancer cachexia. Nutrition 1994;12(1 (Supplement)):S20‐3. - PubMed
Hudson 1994
-
- Hudson EA, Tisdale MJ. Comparison of the effectiveness of eicosapentaenoic acid administered as either the free acid or ethyl ester as an anticachectic and antitumour agent. Prostaglandins, Leukotrienes and essential fatty acids 1994;51:141‐5. - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reporting of randomised clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Jaskowiak 1998
-
- Jaskowiak NT, Alexander Jr. HR. The pathophysiology of cancer cachexia. In: Doyle D, Hanks GWC, MacDonald N editor(s). Oxford Textbook of Palliative Medicine. 2nd Edition. New York: Oxford University Press Inc., 1998:534‐47.
Kyle 2004
-
- Kyle UG, Bosaeus I, Lorenzo AD, Deurenberg P, Elia M, Manual Gomez J, Lilienthal HB, et al. Bioelectrical impedence analysis‐part II: utilization in clinical practice. Clinical Nutrition 2004;5:1226‐43. - PubMed
Lindgvist 2004
-
- Lindgvist O, Widmark A, Rasmussen BH. Meanings of the phenomenon of fatigue as narrated by 4 patients with cancer in palliative care. Cancer Nursing 2004;27:237‐43. - PubMed
Lindsey 1986
-
- Lindsey AM. Cancer cachexia: effects of the disease and its treatment. Seminars in Oncology Nursing 1986;2:19‐29. - PubMed
Nixon 1981
-
- Nixon DW, Lawson DH, Kutner M, Ansley J, Schwarz M, Heymsfield S, et al. Hyperalimentation of the cancer patient with protein‐calorie undernutrition. Cancer Research 1981;41:2038‐45. - PubMed
Ovesen 1993
-
- Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counselling on food intake, body weight, response rate, survival and quality of life in cancer patients undergoing chemotherapy: a prospective randomised study. Journal of Clinical Oncology 1993;11:2043‐9. - PubMed
Smith 1993
Tisdale 1996
-
- Tisdale M. Inhibition of lipolysis and muscle degradation by EPA in cancer cachexia. Nutrition 1996;12(1 (Supplement)):S31‐3. - PubMed
Tisdale 1997
-
- Tisdale MJ. Biology of cachexia. Journal of the National Cacner Institute 1997;89(23):1763‐72. - PubMed
Todorov 1996
-
- Todorov PT, McDevitt TM, Cariuk P. Induction of muscle protein degradation and weight loss by a tumour product. Cancer Research 1996;56:1256‐61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

