Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants
- PMID: 17253571
- PMCID: PMC9004229
- DOI: 10.1002/14651858.CD005948.pub2
Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants
Abstract
Background: Observational studies have shown an association between transiently low thyroid hormone levels in preterm infants in the first weeks of life (transient hypothyroxinaemia) and abnormal neurodevelopmental outcome. Thyroid hormone replacement might prevent this.
Objectives: To determine whether prophylactic thyroid hormones given to preterm infants without congenital hypothyroidism result in clinically important changes in neonatal and long term outcomes.
Search strategy: The standard search strategy of the Neonatal Review Group was used. This included searches of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2006), MEDLINE (1966 - March 2006), EMBASE, PREMEDLINE, and searches of abstracts of conference proceedings, citations of published articles and expert informants.
Selection criteria: All trials using random or quasi-random patient allocation in which prophylactic thyroid hormone treatment was compared to control in premature infants.
Data collection and analysis: Assessment of trial quality, data extraction and synthesis of data, using relative risk (RR) and weighted mean difference (WMD), were performed using standard methods of the Cochrane Collaboration and its Neonatal Review Group.
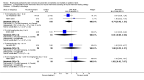
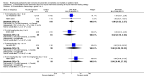
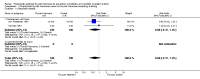
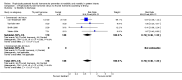
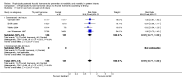
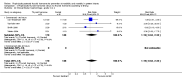
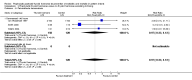
Main results: Four studies enrolling 318 infants were included. All studies enrolled preterm infants on the basis of gestational age criteria. All studies commenced treatment in the first 48 hours, but used different regimens, dose and durations of treatment. All four studies used thyroxine (T4). Valerio 2004 incorporated one arm with an early short course of T3, then T4 for 6 weeks. Only two studies with neurodevelopmental follow-up were of good methodology (van Wassenaer 1997; Vanhole 1997). All studies were small with the largest (van Wassenaer 1997) enrolling 200 infants.No significant difference was found in neonatal morbidity, mortality or neurodevelopmental outcome in infants who received thyroid hormones compared to control. van Wassenaer 1997 reported no significant difference in abnormal mental development at 6, 12, 24 months (RR 0.67, 95% CI 0.28, 1.56) or five years (RR 0.66, 95% CI 0.22, 1.99) or cerebral palsy assessed at five years (RR 0.72, 95% CI 0.28, 1.84). Meta-analysis of two studies (van Wassenaer 1997, Vanhole 1997) found no significant difference in the Bayley MDI (WMD -1.14, 95% CI -5.46, 3.19) and PDI (WMD 0.22, 95% CI -4.80, 5.24) at 7 - 12 months. van Wassenaer 1997 reported no significant difference in the Bayley MDI (MD -3.50, 95% CI -11.21, 4.21) and PDI (MD 3.10, 95% CI -3.31, 9.51) at 24 months, IQ scores at 5 years (MD -2.10, 95% CI -7.91, 3.71) and children in special schooling at 10 years (RR 0.88, 95% CI 0.43, 1.83). Meta-analysis of all four trials found no significant difference in mortality to discharge (typical RR 0.76, 95% CI 0.46 to 1.24). van Wassenaer 1997 reported no significant difference in death or cerebral palsy at five years (RR 0.70, 95% CI 0.43 to 1.14). No significant differences were reported for neonatal morbidities, including the need for mechanical ventilation, duration of mechanical ventilation, air leak, CLD in survivors at 28 days or 36 weeks, intraventricular haemorrhage, severe intraventricular haemorrhage, periventricular leucomalacia, patent ductus arteriosus, sepsis, necrotising enterocolitis or retinopathy of prematurity.
Authors' conclusions: This review does not support the use of prophylactic thyroid hormones in preterm infants to reduce neonatal mortality, neonatal morbidity or improve neurodevelopmental outcomes. An adequately powered clinical trial of thyroid hormone supplementation with the goal of preventing the postnatal nadir of thyroid hormone levels seen in very preterm infants is required.
Conflict of interest statement
None.
Figures



































































































Update of
References
References to studies included in this review
Smith 2000 {published data only}
-
- Smith LM, Leake RD, Berman N, Villanueva S, Brasel JA. Postnatal thyroxine supplementation in infants less than 32 weeks' gestation: effects on pulmonary morbidity. Journal of Perinatology 2000;20:427‐31. - PubMed
Valerio 2004 {published data only}
-
- Valerio PG, Wassenaer AG, Kok JH. A randomized, masked study of T3 plus T4 administration in preterm infants less than 28 weeks of gestational age: hormonal and clinical effects. Pediatric Research. 2002; Vol. 51:125A. - PubMed
-
- Valerio PG, Wassenaer AG, Vijlder JJ, Kok JH. A randomized, masked study of triiodothyronine plus thyroxine administration in preterm infants less than 28 weeks of gestational age: hormonal and clinical effects. Pediatric Research 2004;55:248‐53. - PubMed
van Wassenaer 1997 {published and unpublished data}
-
- Briet JM, Wassenaer AG, Dekker FW, Vijlder JJ, Baar A, Kok JH. Neonatal thyroxine supplementation in very preterm children: developmental outcome evaluated at early school age. Pediatrics 2001;107:712‐8. - PubMed
-
- Briet JM, Wassenaer AG, Baar A, Dekker FW, Kok JH. Evaluation of the effect of thyroxine supplementation on behavioural outcome in very preterm infants. Developmental Medicine and Child Neurology 1999;41:87‐93. - PubMed
-
- Smit BJ, Kok JH, Vries LS, Wassenaer AG, Dekker FW, Ongerboer de Visser BW. Somatosensory evoked potentials in very preterm infants in relation to L‐thyroxine supplementation. Pediatrics 1998;101:865‐9. - PubMed
-
- Smit BJ, Kok JH, Vries LS, Wassenaer AG, Dekker FW, Ongerboer de Visser BW. Motor nerve conduction velocity in very preterm infants in relation to L‐thyroxine supplementation. Journal of Pediatrics 1998;132:64‐9. - PubMed
-
- Wassenaer AG, Kok JH, Briet JM, Pijning AM, Vijlder JJ. Thyroid function in very preterm newborns: possible implications. Thyroid 1999;9:85‐91. - PubMed
Vanhole 1997 {published and unpublished data}
-
- Vanhole C, Aerssens P, Devlieger H, Zegher F. L‐Thyroxine treatment of preterm newborns. Pediatric Research 1996;40:555. - PubMed
-
- Vanhole C, Aerssens P, Naulaers G, Casneuf A, Devlieger H, Berghe G, Zegher F. L‐thyroxine treatment of preterm newborns: clinical and endocrine effects. Pediatric Research 1997;42:87‐92. - PubMed
References to studies excluded from this review
Amato 1988 {published data only}
-
- Amato M, Pasquier S, Carasso A, Muralt G. Postnatal thyroxine administration for idiopathic respiratory distress syndrome in preterm infants. Hormone Research 1988;29:27‐30. - PubMed
Amato 1989 {published data only}
-
- Amato M, Guggisberg C, Schneider H. Postnatal triiodothyronine replacement and respiratory distress syndrome of the preterm infant. Hormone Research 1989;32(5‐6):213‐7. - PubMed
Bettendorf 2000 {published data only}
-
- Bettendorf M, Schmidt KG, Grulich‐Henn J, Ulmer HE, Heinrich UE. Tri‐iodothyronine treatment in children after cardiac surgery: a double‐blind, randomised, placebo‐controlled study. Lancet 2000;356:529‐34. - PubMed
Biswas 2003 {published data only}
-
- Biswas S, Buffery J, Enoch H, Bland JM, Walters D, Markiewicz M. A longitudinal assessment of thyroid hormone concentrations in preterm infants younger than 30 weeks' gestation during the first 2 weeks of life and their relationship to outcome. Pediatrics 2002;109:222‐7. - PubMed
-
- Biswas S. Buffery J. Enoch H. Bland M. Markiewicz M. Walters D. Pulmonary effects of triiodothyronine (T3) and hydrocortisone (HC) supplementation in preterm infants less than 30 weeks gestation: results of the THORN trial‐‐thyroid hormone replacement in neonates. Pediatric Research 2003;53:48‐56. - PubMed
Cassio A 2003 {published data only}
-
- Cassio A, Cacciari E, Cicognani A, Damiani G, Missiroli G, Corbelli E, Balsamo A, Bal M, Gualandi S. Treatment for congenital hypothyroidism: thyroxine alone or thyroxine plus triiodothyronine?. Pediatrics 2003;111:1055‐60. - PubMed
Chowdhry 1984 {published and unpublished data}
-
- Chowdhry P, Scanlon JW, Auerbach R, Abbassi V. Results of controlled double‐blind study of thyroid replacement in very low‐birth‐weight premature infants with hypothyroxinemia. Pediatrics 1984;73:301‐5. - PubMed
Chowdhury 2001 {published data only}
-
- Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. Journal of Thoracic & Cardiovascular Surgery 2001;122:1023‐5. - PubMed
Eggermont 1984 {published data only}
-
- Eggermont E, Vanderschueren Lodeweyckx M, Nayer P, Smeets E, Vanacker G, Cornette C, Jaeken J, Devlieger H, Eeckels R, Beckers C. The thyroid‐system function in preterm infants of postmenstrual ages of 31 weeks or less: evidence for a "transient lazy thyroid system". Helvetica Paediatrica Acta 1984;39:209‐22. - PubMed
Schonberger 1981 {published data only}
-
- Schonberger W, Grimm W, Emmrich P, Gempp W. Reduction of mortality rate in premature infants by substitution of thyroid hormones. European Journal of Pediatrics 1981;135:245‐53. - PubMed
Selva 2002 {published data only}
-
- Selva KA, Mandel SH, Rien L, Sesser D, Miyahira R, Skeels M, Nelson JC, Lafranchi SH. Initial treatment dose of L‐thyroxine in congenital hypothyroidism. Journal of Pediatrics 2002;141:786‐92. - PubMed
van Wassenaer 1993 {published data only}
-
- Wassenaer AG, Kok JH, Endert E, Vulsma T, Vijlder JJ. Thyroxine administration to infants of less than 30 weeks' gestational age does not increase plasma triiodothyronine concentrations. Acta Endocrinologica 1993;129:139‐46. - PubMed
References to ongoing studies
Golombek {unpublished data only}
-
- Hypothyroxemia trial. Ongoing study To be announced.
Additional references
Ballabio 1989
-
- Ballabio M, Nicolini U, Jowett T, Ruiz de Elvira MC, Ekins RP, Rodeck CH. Maturation of thyroid function in normal human foetuses. Clinical Endocrinology 1989;31:565‐71. - PubMed
Belet 2003
-
- Belet N, Imdat H, Yanik F, Kucukoduk S. Thyroid function tests in preterm infants born to preeclamptic mothers with placental insufficiency. Journal of Pediatric Endocrinology 2003;16:1131‐5. - PubMed
Beranl 1995
-
- Beranl J, Nunez J. Thyroid hormones and brain development. European Journal of Endocrinology 1995;133:390‐8. - PubMed
Den Ouden 1996
-
- Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove‐Vanhorick SP. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatric Research 1996;39:142‐5. - PubMed
Filippi 2004
-
- Filippi L, Cecchi A, Tronchin M, Dani C, Pezzati M, Seminara S, et al. Dopamine infusion and hypothyroxinaemia in very low birth weight preterm infants. European Journal of Pediatrics 2004;163:7‐13. - PubMed
Frank 1996
-
- Frank JE, Faix JE, Hermos RJ, Mullaney DM, Rojan DA, Mitchell ML, et al. Thyroid function in very low birth weight infants: effects on hypothyroidism screening. Journal of Pediatrics 1996;128:548‐54. - PubMed
Franklin 1986
Hsu 1999
-
- Hsu CH, Chang JH, Lee YJ, Hung HY, Kao HA, Huang FY. Thyroid function in the sick very low‐birth‐weight infants. Acta Paediatrica Taiwanica 1999;40:237‐42. - PubMed
Kantor Herring 2003
-
- Kantor Herring MJ, Leef KH, Locke RG, Stefano JL, Bartoshesky L, Paul DA. Are perinatal risk factors helpful in predicting and optimizing treatment strategies for transient hypothyroxinemia in very‐low‐birth‐weight infants?. American Journal of Perinatology 2003;20:333‐9. - PubMed
Kooistra 1994
-
- Kooistra L, Laane C, Vulsma T, Schellekens JMH, Meere JJ, Kalverboer AF. Motor and cognitive development in children with congenital hypothyroidism: a long‐term evaluation of the effects of neonatal treatment. Journal of Pediatrics 1994;124:903‐9. - PubMed
Linder 1997
-
- Linder N, Davidovitch N, Reichman B, Kuint J, Lubin D, Meyerovitch J, et al. Topical iodine‐containing antiseptics and subclinical hypothyroidism in preterm infants. Journal of Pediatrics 1997;131:434‐9. - PubMed
Lorenz 1998
-
- Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Archives of Pediatric and Adolescent Medicine 1998;152:425‐35. - PubMed
Lucas 1988
Lucas 1996
Meijer 1992
Oden 2002
-
- Oden J, Freemark M. Thyroxine supplementation in preterm infants: critical analysis. Current Opinion in Pediatrics 2002;14:447‐52. - PubMed
Osborn 2007a
Osborn 2007b
Paneth 1998
-
- Paneth N. Does transient thyroxinemia cause abnormal neurodevelopment in premature infants?. Clinics in Perinatology 1998;25:627‐43. - PubMed
Paul 1998
-
- Paul DA, Leef KH, Stefano JL, Bartoshesky L. Low serum thyroxine on initial newborn screening is associated with intraventricular hemorrhage and death in very low birth weight infants. Pediatrics 1998;101:903‐7. - PubMed
Paul 2000
-
- Paul DA, Leef KH, Stefano JL, Bartoshesky L. Thyroid function in very‐low‐birth‐weight infants with intraventricular hemorrhage. Clinics in Pediatrics 2000;39:651‐6. - PubMed
Porterfield 1993
-
- Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development ‐ current perspectives. Endocrine Reviews 1993;14:94‐106. - PubMed
Radunovic 1991
-
- Radunovic N, Dumez Y, Nastic D, Mandelbrot L, Dommergues M. Thyroid function in fetus and mother during the second half of normal pregnancy. Biology of the Neonate 1991;59:139‐48. - PubMed
Reuss 1996
-
- Reuss ML, Paneth N, Pinto‐Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. New England Journal of Medicine 1996;334:821‐7. - PubMed
Reuss 1997
Rooman 1996
-
- Rooman RP, Du Caju MV, Beeck LO, Docx M, Reempts P, Acker KJ. Low thyroxinaemia occurs in the majority of very preterm newborns. European Journal of Pediatrics 1996;155:211‐5. - PubMed
Tahirovic 1994
-
- Tahirovic HF. Transient hypothyroxinemia in neonates with birth asphyxia delivered by emergency cesarean section. Journal of Pediatric Endocrinology 1994;7:39‐41. - PubMed
Thorpe‐Beeston 1991
-
- Thorpe‐Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM. Maturation of the secretion of thyroid hormone and thyroid stimulating hormone in the fetus. New England Journal of Medicine 1991;324:532‐6. - PubMed
Uhrmann 1981
van Wassenaer 1997
-
- Wassenaer AG, Kok JH, Dekker FW, Vijlder JJ. Thyroid function in very preterm infants: influences of gestational age and disease. Pediatric Research 1997;42:604‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

