Interstrand cross-links generated by abasic sites in duplex DNA
- PMID: 17253689
- PMCID: PMC2812894
- DOI: 10.1021/ja067294u
Interstrand cross-links generated by abasic sites in duplex DNA
Figures



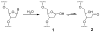

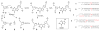
References
-
-
For examples of abasic sites stemming from DNA alkylation: see: Gates KS, Nooner T, Dutta S. Chem Res Toxicol. 2004;17:839–856.Nooner T, Dutta S, Gates KS. Chem Res Toxicol. 2004;17:942–949.
-
-
- Freese E, Cashel M. Biochim Biophys Acta. 1964;91:67–77. - PubMed
-
- Burnotte J, Verly WG. Biochim Biophys Acta. 1972;262:449–452. - PubMed
- Goffin C, Verly WG. FEBS Lett. 1983;161:140–144. - PubMed
- Goffin C, Verly WG. Biochim Biophys Acta. 1984;783:1–5. - PubMed
- Mittal A, Musarrat J. Med Sci Res. 1990;18:633–635.
- Prakash AS, Gibson NW. Carcinogenesis. 1992;13:425–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

