Effect of mutations in the cytochrome b ef loop on the electron-transfer reactions of the Rieske iron-sulfur protein in the cytochrome bc1 complex
- PMID: 17253777
- PMCID: PMC2527182
- DOI: 10.1021/bi062094g
Effect of mutations in the cytochrome b ef loop on the electron-transfer reactions of the Rieske iron-sulfur protein in the cytochrome bc1 complex
Abstract
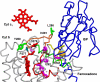
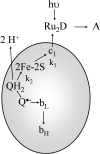
Long-range movement of the Rieske iron-sulfur protein (ISP) between the cytochrome (cyt) b and cyt c1 redox centers plays a key role in electron transfer within the cyt bc1 complex. A series of 21 mutants in the cyt b ef loop of Rhodobacter sphaeroides cyt bc1 were prepared to examine the role of this loop in controlling the capture and release of the ISP from cyt b. Electron transfer in the cyt bc1 complex was studied using a ruthenium dimer to rapidly photo-oxidize cyt c1 within 1 mus and initiate the reaction. The rate constant for electron transfer from the Rieske iron-sulfur center [2Fe2S] to cyt c1 was k1 = 60 000 s-1. Famoxadone binding to the Qo site decreases k1 to 5400 s-1, indicating that a conformational change on the surface of cyt b decreases the rate of release of the ISP from cyt b. The mutation I292A on the surface of the ISP-binding crater decreased k1 to 4400 s-1, while the addition of famoxadone further decreased it to 3000 s-1. The mutation L286A at the tip of the ef loop decreased k1 to 33 000 s-1, but famoxadone binding caused no further decrease, suggesting that this mutation blocked the conformational change induced by famoxadone. Studies of all of the mutants provide further evidence that the ef loop plays an important role in regulating the domain movement of the ISP to facilitate productive electron transfer and prevent short-circuit reactions.
Figures





References
-
- Trumpower BL, Gennis RB. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu. Rev. Biochem. 1994;63:675–716. - PubMed
-
- Trumpower BL. The Protonmotive Q Cycle. Energy Transduction by Coupling of Proton Translocation to Electron Transfer by the Cytochrome bc1 Complex. J. Biol. Chem. 1990;265:11409–11412. - PubMed
-
- Xia D, Yu C-A, Kim H, Xia J-Z, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal Structure of the Cytochrome bc1 Complex from Bovine Heart Mitochondria. Science. 1997;277:60–66. - PubMed
-
- Zhang Z, Huang L, Shulmeister VM, Chi Y-I, Kim KK, Hung L-W, Crofts AR, Berry EA, Kim S-H. Electron Transfer by Domain Movement in Cytochrome bc1. Nature. 1998;392:677–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

