Structural and functional analysis of the pyocyanin biosynthetic protein PhzM from Pseudomonas aeruginosa
- PMID: 17253782
- PMCID: PMC2572083
- DOI: 10.1021/bi6024403
Structural and functional analysis of the pyocyanin biosynthetic protein PhzM from Pseudomonas aeruginosa
Abstract
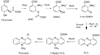
Pyocyanin is a biologically active phenazine produced by the human pathogen Pseudomonas aeruginosa. It is thought to endow P. aeruginosa with a competitive growth advantage in colonized tissue and is also thought to be a virulence factor in diseases such as cystic fibrosis and AIDS where patients are commonly infected by pathogenic Pseudomonads due to their immunocompromised state. Pyocyanin is also a chemically interesting compound due to its unusual oxidation-reduction activity. Phenazine-1-carboxylic acid, the precursor to the bioactive phenazines, is synthesized from chorismic acid by enzymes encoded in a seven-gene cistron in P. aeruginosa and in other Pseudomonads. Phenzine-1-carboxylic acid is believed to be converted to pyocyanin by the sequential actions of the putative S-adenosylmethionine-dependent N-methyltransferase PhzM and the putative flavin-dependent hydroxylase PhzS. Here we report the 1.8 A crystal structure of PhzM determined by single anomalous dispersion. Unlike many methyltransferases, PhzM is a dimer in solution. The 36 kDa PhzM polypeptide folds into three domains. The C-terminal domain exhibits the alpha/beta-hydrolase fold typical of small molecule methyltransferases. Two smaller N-terminal domains form much of the dimer interface. Structural alignments with known methyltransferases show that PhzM is most similar to the plant O-methyltransferases that are characterized by an unusual intertwined dimer interface. The structure of PhzM contains no ligands, and the active site is open and solvent-exposed when compared to structures of similar enzymes. In vitro experiments using purified PhzM alone demonstrate that it has little or no ability to methylate phenzine-1-carboxylic acid. However, when the putative hydroxylase PhzS is included, pyocyanin is readily produced. This observation suggests that a mechanism has evolved in P. aeruginosa that ensures efficient production of pyocyanin via the prevention of the formation and release of an unstable and potentially deleterious intermediate.
Figures



References
-
- Laursen JB, Nielsen J. Phenazine Natural Products: Biosynthesis, Synthetic Analogues, and Biological Activity. Chem. Rev. 2004;104:1663–1686. - PubMed
-
- Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J. Antimicrob. Chemother. 2003;52:668–674. - PubMed
-
- Reszka KJ, Denning GM, Britigan BE. Photosensitized oxidation and inactivation of pyocyanin, a virulence factor of Pseudomonas aeruginosa. Photochem. Photobiol. 2006;82:466–473. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

