Demonstration of infectious salmon anaemia virus (ISAV) endocytosis in erythrocytes of Atlantic salmon
- PMID: 17254352
- PMCID: PMC1793955
- DOI: 10.1186/1743-422X-4-13
Demonstration of infectious salmon anaemia virus (ISAV) endocytosis in erythrocytes of Atlantic salmon
Abstract
Infectious salmon anaemia (ISA) virus (ISAV) is a fish orthomyxovirus that has recently been assigned to the new genus Isavirus within the family Orthomyxoviridae. It possesses the major functional characteristics of the virus family including haemagglutinating, receptor destroying enzyme (RDE), and fusion activities associated with the virion surface proteins. It is generally accepted that ISAV agglutinates erythrocytes of several fish species and that the ISAV RDE activity dissolves this haemagglutination reaction except for Atlantic salmon (Salmo salar) erythrocytes. We used electron microscopy to examine the physical interaction between ISAV and erythrocytes from Atlantic salmon and rainbow trout (Oncorhynchus mykiss) during haemagglutination. We present evidence that ISAV enters into Atlantic salmon erythrocytes. Atlantic salmon erythrocytes incubated with ISAV for 4 hours showed endocytosis of the virus particles, which is consistent with virus infection. These observations suggest that the lack of dissolution of ISAV-induced haemagglutination of Atlantic salmon erythrocytes favours virus infection of the erythrocytes. Moreover, such a haemagglutination-infection phenotype is fundamentally different from haemagglutination by avian and mammalian orthomyxoviruses, and is indicative of a different pathogenesis for the fish orthomyxovirus.
Figures
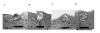
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

