FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis
- PMID: 17254969
- PMCID: PMC1855089
- DOI: 10.1016/j.cell.2006.12.029
FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis
Abstract
Activated phosphoinositide 3-kinase (PI3K)-AKT signaling appears to be an obligate event in the development of cancer. The highly related members of the mammalian FoxO transcription factor family, FoxO1, FoxO3, and FoxO4, represent one of several effector arms of PI3K-AKT signaling, prompting genetic analysis of the role of FoxOs in the neoplastic phenotypes linked to PI3K-AKT activation. While germline or somatic deletion of up to five FoxO alleles produced remarkably modest neoplastic phenotypes, broad somatic deletion of all FoxOs engendered a progressive cancer-prone condition characterized by thymic lymphomas and hemangiomas, demonstrating that the mammalian FoxOs are indeed bona fide tumor suppressors. Transcriptome and promoter analyses of differentially affected endothelium identified direct FoxO targets and revealed that FoxO regulation of these targets in vivo is highly context-specific, even in the same cell type. Functional studies validated Sprouty2 and PBX1, among others, as FoxO-regulated mediators of endothelial cell morphogenesis and vascular homeostasis.
Figures
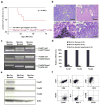


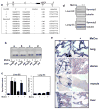

Comment in
-
FoxOs in tumor suppression and stem cell maintenance.Cell. 2007 Jan 26;128(2):235-7. doi: 10.1016/j.cell.2007.01.009. Cell. 2007. PMID: 17254960 Review.
References
-
- Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–199. - PubMed
-
- Benezra R. Role of Id proteins in embryonic and tumor angiogenesis. Trends Cardiovasc Med. 2001;11:237–241. - PubMed
-
- Biggs WH, 3rd, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome. 2001;12:416–425. - PubMed
-
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

