Early Cambrian origin of modern food webs: evidence from predator arrow worms
- PMID: 17254986
- PMCID: PMC2197202
- DOI: 10.1098/rspb.2006.3761
Early Cambrian origin of modern food webs: evidence from predator arrow worms
Abstract
Although palaeontological evidence from exceptional biota demonstrates the existence of diverse marine communities in the Early Cambrian (approx. 540-520 Myr ago), little is known concerning the functioning of the marine ecosystem, especially its trophic structure and the full range of ecological niches colonized by the fauna. The presence of a diverse zooplankton in Early Cambrian oceans is still an open issue. Here we provide compelling evidence that chaetognaths, an important element of modern zooplankton, were present in the Early Cambrian Chengjiang biota with morphologies almost identical to Recent forms. New information obtained from the lowermost Cambrian of China added to previous studies provide convincing evidence that protoconodont-bearing animals also belonged to chaetognaths. Chaetognaths were probably widespread and diverse in the earliest Cambrian. The obvious raptorial function of their circumoral apparatuses (grasping spines) places them among the earliest active predator metazoans. Morphology, body ratios and distribution suggest that the ancestral chaetognaths were planktonic with possible ecological preferences for hyperbenthic niches close to the sea bottom. Our results point to the early introduction of prey-predator relationships into the pelagic realm, and to the increase of trophic complexity (three-level structure) during the Precambrian-Cambrian transition, thus laying the foundations of present-day marine food chains.
Figures
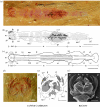

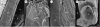

References
-
- Andres D. Strukturen, Apparate und Phylogenie primitiver Conodonten. Palaeontographica Abt. A. 1988;200:105–152.
-
- Azmi, R. J. 1996 Evidence for soft tissue basal support in earliest Cambrian protoconodonts from the Lesser Himalaya: conodont function and affinity. In Contributions to the 15th Indian Colloquium on Micropalaeontology and Stratigraphy, Dehra Dun (ed. J. Pandey, R. J. Azmi, A. Bhandari, & A. Dave), pp. 457–463. Dehra Dun, India: Wadia Institute of Himalayan Geology.
-
- Bengtson S. The structure of some Middle Cambrian conodonts, and the early evolution of conodont structure and function. Lethaia. 1976;9:185–206.
-
- Bengtson S. The early history of the Conodonta. Fossils and Strata. 1983;15:5–19.
-
- Bone Q, Duvert M. Locomotion and buoyancy. In: Bone Q, Kapp H, Pierrot-Bults A.C, editors. The biology of chaetognaths. Oxford University Press; Oxford, UK: 1991. pp. 32–44.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

