Useful signals from motor cortex
- PMID: 17255162
- PMCID: PMC2151362
- DOI: 10.1113/jphysiol.2006.126698
Useful signals from motor cortex
Abstract
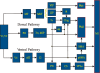
Historically, the motor cortical function has been explained as a funnel to muscle activation. This invokes the idea that motor cortical neurons, or 'upper motoneurons', directly cause muscle contraction just like spinal motoneurons. Thus, the motor cortex and muscle activity are inextricably entwined like a puppet master and his marionette. Recently, this concept has been challenged by current experimentation showing that many behavioural aspects of action are represented in motor cortical activity. Although this activity may still be related to muscle activation, the relation between the two is likely to be indirect and complex, whereas the relation between cortical activity and kinematic parameters is simple and robust. These findings show how to extract useful signals that help explain the underlying process that generates behaviour and to harness these signals for potentially therapeutic applications.
Figures



















References
-
- Bernstein NA. The Coordination and Regulation of Movements. Oxford: Pergamon Press; 1967.
-
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. - PubMed
-
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. - PubMed
-
- Ferrier D. Experiments on the brains of monkeys. Proc R Soc London (Biol) 1875;23:409–430.
-
- Fitts PM, Denninger RL. S-R compatibility: correspondence among paired elements within stimulus and response codes. J Exp Psychol. 1954;48:483–492. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

