Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors
- PMID: 17255166
- PMCID: PMC2075437
- DOI: 10.1113/jphysiol.2006.125336
Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors
Abstract
The retrotrapezoid nucleus (RTN) contains CO(2)-activated interneurons with properties consistent with central respiratory chemoreceptors. These neurons are glutamatergic and express the transcription factor Phox2b. Here we tested whether RTN neurons receive an input from slowly adapting pulmonary stretch receptors (SARs) in halothane-anaesthetized ventilated rats. In vagotomized rats, RTN neurons were inhibited to a variable extent by stimulating myelinated vagal afferents using the lowest intensity needed to inhibit the phrenic nerve discharge (PND). In rats with intact vagus nerves, RTN neurons were inhibited, also to a variable extent, by increasing positive end-expiratory pressure (PEEP; 2-6 cmH(2)O). The cells most sensitive to PEEP were inhibited during each lung inflation at rest and were instantly activated by stopping ventilation. Muscimol (GABA-A agonist) injection in or next to the solitary tract at area postrema level desynchronized PND from ventilation, eliminated the lung inflation-synchronous inhibition of RTN neurons and their steady inhibition by PEEP but did not change their CO(2) sensitivity. Muscimol injection into the rostral ventral respiratory group eliminated PND but did not change RTN neuron response to either lung inflation, PEEP increases, vagal stimulation or CO(2). Generalized glutamate receptor blockade with intracerebroventricular (i.c.v.) kynurenate eliminated PND and the response of RTN neurons to lung inflation but did not change their CO(2) sensitivity. PEEP-sensitive RTN neurons expressed Phox2b. In conclusion, RTN chemoreceptors receive an inhibitory input from myelinated lung stretch receptors, presumably SARs. The lung input to RTN may be di-synaptic with inhibitory pump cells as sole interneurons.
Figures






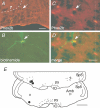
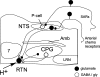
Comment in
-
Reflexively inhibiting respiratory drive.J Physiol. 2007 Apr 1;580(Pt 1):3. doi: 10.1113/jphysiol.2007.130500. Epub 2007 Feb 22. J Physiol. 2007. PMID: 17317738 Free PMC article. No abstract available.
References
-
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–479. - PubMed
-
- Bodineau L, Frugière A, Marlot D, Wallois F. Connections between retrotrapezoid nucleus and nucleus tractus solitarii in cat. Neurosci Lett. 2000a;280:111–114. - PubMed
-
- Bodineau L, Frugiere A, Marlot D, Wallois F. Effect of hypoxia on the activity of respiratory and non-respiratory modulated retrotrapezoid neurons of the cat. Auton Neurosci. 2000b;86:70–77. - PubMed
-
- Coleridge HM, Coleridge JC. Afferent innervation of lungs, airways, and pulmonary artery. In: Zucker IH, Gilmore JP, editors. Reflex Control of the Circulation. Boca Raton: CRC Press; 2001. pp. 579–607.
-
- Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol. 1990;258:L33–L44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

