Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements
- PMID: 17255169
- PMCID: PMC2075553
- DOI: 10.1113/jphysiol.2006.123075
Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements
Abstract
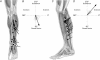
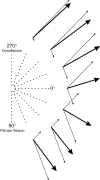
The aim of this study was to analyse the directional coding of two-dimensional limb movements by cutaneous afferents from skin areas covering a multidirectional joint, the ankle. The activity of 89 cutaneous afferents was recorded in the common peroneal nerve, and the mean discharge frequency of each unit was measured during the outward phase of ramp and hold movements imposed in 16 different directions. Forty-two afferents responded to the movements in the following decreasing order (SA2, n = 24/27; FA2, n = 13/17; FA1, n = 3/24; SA1, n = 2/21). All the units activated responded to a specific range of directions, defining their 'preferred sector', within which their response peaked in a given direction, their 'preferred direction'. Based on the distribution of the preferred directions, two populations of afferents, and hence two skin areas were defined: the anterior and the external lateral parts of the leg. As the directional tuning of each population was cosine shaped, the neuronal population vector model was applied and found to efficiently describe the movement direction encoded by cutaneous afferents, as it has been previously reported for muscle afferents. The responses of cutaneous afferents were then considered with respect to those of the afferents from the underlying muscles, which were previously investigated, and an almost perfect matching of directional sensitivity was observed. It is suggested that the common movement-encoding characteristics exhibited by cutaneous and muscle afferents, as early as the peripheral level, may facilitate the central co-processing of their feedbacks subserving kinaesthesia.
Figures





References
-
- Albert F, Bergenheim M, Ribot-Ciscar E, Roll JP. The Ia afferent feedback of a given movement evokes the illusion of the same movement when returned to the subject via muscle tendon vibration. Exp Brain Res. 2006;172:163–174. - PubMed
-
- Batschelet E. Circular Statistics in Biology. London: Academic Press; 1981.
-
- Bergenheim M, Ribot-Ciscar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans. I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res. 2000;134:301–310. - PubMed
-
- Bergenheim M, Roll JP, Ribot-Ciscar E. Microneurography in humans. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Berlin Heidelberg New York: Springer; 1999. pp. 801–819.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

