Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics
- PMID: 17255279
- PMCID: PMC4030524
- DOI: 10.1158/1078-0432.CCR-06-1576
Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics
Abstract
Purpose: Allogeneic glioma cell lines that are partially matched to the patient at class I human leukocyte antigen (HLA) loci and that display tumor-associated antigens (TAA) or antigenic precursors [tumor antigen precursor proteins (TAPP)] could be used for generating whole tumor cell vaccines or, alternatively, for extraction of TAA peptides to make autologous dendritic cell vaccines.
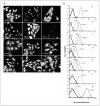
Experimental design: Twenty human glioma cell lines were characterized by molecular phenotyping and by flow cytometry for HLA class I antigen expression. Twelve of the 20 cell lines, as well as analyses of freshly resected glioma tissues, were further characterized for protein and/or mRNA expression of 16 tumor antigen precursor proteins or TAA.
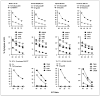
Results: These 20 human glioma cell lines potentially cover 77%, 85%, and 78% of the U.S. Caucasian population at HLA-A, HLA-B, and HLA-C alleles, respectively. All cells exhibited multiple TAA expressions. Most glioma cells expressed antigen isolated from immunoselected melanoma-2 (Aim-2), B-cyclin, EphA2, GP100, beta1,6-N-acetylglucosaminyltransferase V (GnT-V), IL13Ralpha2, Her2/neu, hTert, Mage, Mart-1, Sart-1, and survivin. Real-time PCR technology showed that glioblastoma specimens expressed most of the TAA as well. Tumor-infiltrating lymphocytes and CD8(+) CTL killed T2 cells when loaded with specific HLA-A2(+) restricted TAA, or gliomas that were both HLA-A2(+) and also positive for specific TAA (Mart-1, GP100, Her2/neu, and tyrosinase) but not those cells negative for HLA-A2 and/or lacking the specific epitope.
Conclusions: These data provide proof-in-principle for the use of allogeneic, partially HLA patient-matched glioma cells for vaccine generation or for peptide pulsing with allogeneic glioma cell extracts of autologous patient dendritic cells to induce endogenous CTL in brain tumor patients.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Mahaley MS, Jr., Bigner DD, Dudka LF, et al. Immunobiology of primary intracranial tumors. Part 7. Active immunization of patients with anaplastic human glioma cells: a pilot study. J Neurosurg. 1983;59:201–7. - PubMed
-
- Plautz GE, Miller DW, Barnet GH, et al. T Cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6:2209–18. - PubMed
-
- Hayes RL, Arbit E, Odaimi M, et al. Adoptive cellular immunotherapy for the treatment of malignant gliomas. Crit Rev Oncol Hematol. 2001;39:31–42. - PubMed
-
- Quattrocchi KB, Miller CH, Cush S, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45:141–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

