Osteoclasts: what do they do and how do they do it?
- PMID: 17255310
- PMCID: PMC1851862
- DOI: 10.2353/ajpath.2007.060834
Osteoclasts: what do they do and how do they do it?
Abstract
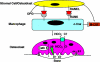
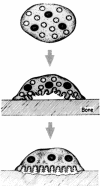
As Americans live longer, degenerative skeletal diseases, such as osteoporosis, become increasingly prevalent. Regardless of cause, osteoporosis reflects a relative enhancement of osteoclast activity. Thus, this unique bone resorptive cell is a prominent therapeutic target. A number of key observations provide insights into the mechanisms by which precursors commit to the osteoclast phenotype and how the mature cell degrades bone. The osteoclast is a member of the monocyte/macrophage family that differentiates under the aegis of two critical cytokines, namely RANK ligand and M-CSF. Tumor necrosis factor (TNF)-alpha also promotes osteoclastogenesis, particularly in states of inflammatory osteolysis such as that attending rheumatoid arthritis. Once differentiated, the osteoclast forms an intimate relationship with the bone surface via the alphavbeta3 integrin, which transmits matrix-derived, cytoskeleton-organizing, signals. These integrin-transmitted signals include activation of the associated proteins, c-src, syk, Vav3, and Rho GTPases. The organized cytoskeleton generates an isolated microenvironment between the cell's plasma membrane and the bone surface in which matrix mineral is mobilized by the acidic milieu and organic matrix is degraded by the lysosomal protease, cathepsin K. This review focuses on these and other molecules that mediate osteoclast differentiation or function and thus serve as candidate anti-osteoporosis therapeutic targets.
Figures


References
-
- Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463. - PubMed
-
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. - PubMed
-
- Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975;190:784–785. - PubMed
-
- Walker DG. Spleen cells transmit osteopetrosis in mice. Science. 1975;190:785–787. - PubMed
-
- Coccia PF, Krivit W, Cervenka J, Clawson C, Kersey JH, Kim TH, Nesbit ME, Ramsay NK, Warkentin PI, Teitelbaum SL, Kahn AJ, Brown DM. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med. 1980;302:701–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

