Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis
- PMID: 17255343
- PMCID: PMC1851877
- DOI: 10.2353/ajpath.2007.060761
Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis
Abstract
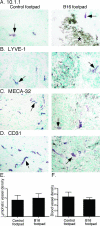
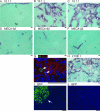
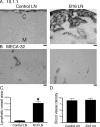
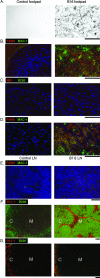
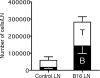
Lymphangiogenesis is associated with human and murine cancer metastasis, suggesting that lymphatic vessels are important for tumor dissemination. Lymphatic vessel alterations were examined using B16-F10 melanoma cells implanted in syngeneic C57Bl/6 mice, which form tumors metastasizing to draining lymph nodes and subsequently to the lungs. Footpad tumors showed no lymphatic or blood vessel growth; however, the tumor-draining popliteal lymph node featured greatly increased lymphatic sinuses. Lymph node lymphangiogenesis began before melanoma cells reached draining lymph nodes, indicating that primary tumors induce these alterations at a distance. Lymph flow imaging revealed that nanoparticle transit was greatly increased through tumor-draining relative to nondraining lymph nodes. Lymph node lymphatic sinuses and lymph flow were increased in mice implanted with unmarked or with foreign antigen-expressing melanomas, indicating that these effects are not due to foreign antigen expression. However, tumor-derived immune signaling could promote lymph node alterations, as macrophages infiltrated footpad tumors, whereas lymphocytes accumulated in tumor-draining lymph nodes. B lymphocytes are required for lymphangiogenesis and increased lymph flow through tumor-draining lymph nodes, as these alterations were not observed in mice deficient for B cells. Lymph node lymphangiogenesis and increased lymph flow through tumor-draining lymph nodes may actively promote metastasis via the lymphatics.
Figures

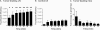

References
-
- Cao Y. Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–743. - PubMed
-
- He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654:3–12. - PubMed
-
- Stacker S, Baldwin M, Achen M. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. - PubMed
-
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. - PubMed
-
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

