TCRzetadim lymphocytes define populations of circulating effector cells that migrate to inflamed tissues
- PMID: 17255353
- PMCID: PMC1939810
- DOI: 10.1182/blood-2006-12-064170
TCRzetadim lymphocytes define populations of circulating effector cells that migrate to inflamed tissues
Abstract
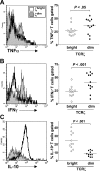
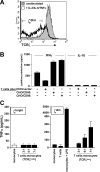
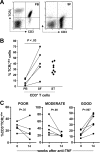
The T-cell receptor zeta (TCRzeta) chain is a master sensor and regulator of lymphocyte responses. Loss of TCRzeta expression has been documented in infectious, inflammatory, and malignant diseases, suggesting that it may serve to limit T-cell reactivity and effector responses at sites of tissue damage. These observations prompted us to explore the relationship between TCRzeta expression and effector function in T cells. We report here that TCRzeta(dim) lymphocytes are enriched for antigen-experienced cells refractory to TCR-induced proliferation. Compared to their TCRzeta(bright) counterparts, TCRzeta(dim) cells share characteristics of differentiated effector T cells but use accessory pathways for transducing signals for inflammatory cytokine gene expression and cell contact-dependent pathways to activate monocytes. TCRzeta(dim) T cells accumulate in inflamed tissues in vivo and have intrinsic migratory activity in vitro. Whilst blocking leukocyte trafficking with anti-TNF therapy in vivo is associated with the accumulation of TCRzeta(dim) T cells in peripheral blood, this T-cell subset retains the capacity to migrate in vitro. Taken together, the functional properties of TCRzeta(dim) T cells make them promising cellular targets for the treatment of chronic inflammatory disease.
Figures
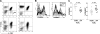



References
-
- Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. - PubMed
-
- Klausner RD, Lippincott-Schwartz J, Bonifacino JS. The T cell antigen receptor: insights into organelle biology. Annu Rev Cell Biol. 1990;6:403–431. - PubMed
-
- Cosson P, Lankford SP, Bonifacino JS, Klausner RD. Membrane protein association by potential intramembrane charge pairs. Nature. 1991;351:414–416. - PubMed
-
- Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. - PubMed
-
- Letourneur F, Klausner RD. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992;255:79–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

