Nuclear factor-kappaB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation
- PMID: 17255561
- PMCID: PMC1899293
- DOI: 10.1164/rccm.200608-1162OC
Nuclear factor-kappaB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation
Abstract
Rationale: Injurious agents often cause less severe injury in neonates as compared with adults.
Objective: We hypothesized that maturational differences in lung inflammation induced by lipopolysaccharide (LPS) may be related to the nature of the nuclear factor (NF)-kappaB complex activated, and the profile of target genes expressed.
Methods: Neonatal and adult mice were injected with intraperitoneal LPS. Lung inflammation was assessed by histology, and apoptosis was determined by TUNEL (terminal deoxynucleotidyl transferase UTP nick-end labeling). The expression of candidate inflammatory and apoptotic mediators was evaluated by quantitative real-time polymerase chain reaction and Western immunoblot.
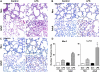
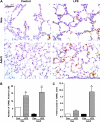
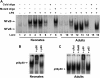
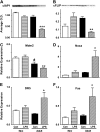
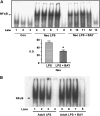
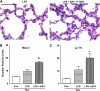
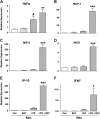
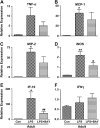
Results: Neonates demonstrated reduced inflammation and apoptosis, 24 hours after LPS exposure, as compared with adults. This difference was associated with persistent activation of NF-kappaB p65p50 heterodimers in the neonates in contrast to early, transient activation of p65p50 followed by sustained activation of p50p50 in the adults. Adults had increased expression of a panel of inflammatory and proapoptotic genes, and repression of antiapoptotic targets, whereas no significant changes in these mediators were observed in the neonates. Inhibition of NF-kappaB activity in the neonates decreased apoptosis, but heightened inflammation, with increased expression of the same inflammatory genes elevated in the adults. In contrast, inhibition of NF-kappaB in the adults resulted in partial suppression of the inflammatory response.
Conclusions: NF-kappaB activation in the neonatal lung is antiinflammatory, protecting against LPS-mediated lung inflammation by repressing similar inflammatory genes induced in the adult.
Figures



References
-
- Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med 2004;141:460–470. - PubMed
-
- Estenssoro E, Dubin A, Laffaire E, Canales H, Saenz G, Moseinco M, Pozo M, Gomez A, Baredes N, Jannello G, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med 2002;30:2450–2456. - PubMed
-
- Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol 1999;163:2217–2225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

