Adipose tissue engineering from human adult stem cells: clinical implications in plastic and reconstructive surgery
- PMID: 17255658
- PMCID: PMC4035042
- DOI: 10.1097/01.prs.0000244840.80661.e7
Adipose tissue engineering from human adult stem cells: clinical implications in plastic and reconstructive surgery
Abstract
Background: Despite certain levels of clinical efficacy, current autografts and synthetic materials for soft-tissue reconstruction and/or augmentation suffer from donor-site morbidity, rupture, dislocation, and volume reduction. Human adult stem cells can self-replicate and differentiate into adipogenic cells in response to appropriate signaling cues. This study investigated the shape and dimension maintenance of engineered adipose tissue from adult human mesenchymal stem cells.
Methods: Human mesenchymal stem cells were isolated from bone marrow of a healthy donor and differentiated into adipogenic cells. Adipocytes derived from these cells were encapsulated in a poly(ethylene glycol)-based hydrogel shaped into a generic cylinder (n = 6 implants), with hydrogel encapsulating human mesenchymal stem cells (n = 6) and cell-free hydrogel (n = 6) as controls. Porous collagen sponges were also used to seed human mesenchymal stem cell-derived adipocytes (n = 6), human mesenchymal stem cells (n = 4), or without cells (n = 4). All poly(ethylene glycol) and collagen constructs were implanted subcutaneously in athymic mice for 4 weeks.
Results: In vivo grafts demonstrated the formation of substantial adipose tissue encapsulating human mesenchymal stem cell-derived adipogenic cells in either poly(ethylene glycol)-based hydrogel or collagen sponge and a lack of adipose tissue formation in cell-free or human mesenchymal stem cell-derived grafts. Engineered adipose tissue in poly(ethylene glycol)-based hydrogel maintained approximately 100 percent of the original dimensions after 4-week in vivo implantation, significantly higher than the approximately 35 to 65 percent volume retention by collagen sponge.
Conclusions: These findings demonstrate that the predefined shape and dimensions of adipose tissue engineered from human mesenchymal stem cells can be maintained after in vivo implantation. These data further indicate the potential for autologous applications in reconstructive and plastic surgery procedures.
Figures



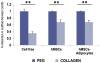


References
-
- Patrick CW, Jr, Chauvin PB, Reece GP. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999;5:139. - PubMed
-
- Beahm EK, Walton RL, Patrick CW., Jr Progress in adipose tissue construct development. Clin Plast Surg. 2003;30:547. - PubMed
-
- Alster TS, West TB. Human-derived and new synthetic injectable materials for soft-tissue augmentation: Current status and role in cosmetic surgery. Plast Reconstr Surg. 2000;105:2515. - PubMed
-
- American Society of Plastic Surgeons (Web site) [Accessed March 26, 2005.]. Available at: http//www.plasticsurgery.org.
-
- Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26:587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

