Sulfation, the up-and-coming post-translational modification: its role and mechanism in protein-protein interaction
- PMID: 17256885
- PMCID: PMC2954651
- DOI: 10.1021/pr060529g
Sulfation, the up-and-coming post-translational modification: its role and mechanism in protein-protein interaction
Abstract
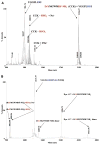
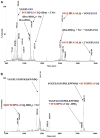
Tyrosine sulfation is a post-translational modification entailing covalent attachment of sulfate to tyrosine residues. It takes place in the trans-Golgi, is necessary for the bioactivity of some proteins, and improves their ability to interact with other proteins. In the present work, we show that a protein containing a sulfated tyrosine with a delocalized negative charge forms a salt bridge with another protein if it has two or more adjacent arginine residues containing positive delocalized charges. These noncovalent complexes are so stable that, when submitted to collision induced dissociation, the peptides forming the complex dissociate. Just one covalent bond fragments, the covalent bond between the tyrosine oxygen and the SO3 sulfur, and is represented by the appearance of a new peak (basic peptide + SO3), suggesting that in some instances covalent bonds will break down before the noncovalent bonds between the arginine guanidinium and SO3 dissociate. The data implies that the dissociation pathway is preferred; however, fragmentation between tyrosine and the sulfate residue is a major pathway.
Figures



Similar articles
-
Histidine, the less interactive cousin of arginine.Eur J Mass Spectrom (Chichester). 2019 Apr;25(2):212-218. doi: 10.1177/1469066718791793. Eur J Mass Spectrom (Chichester). 2019. PMID: 31018697 Free PMC article.
-
The role of phosphorylated residues in peptide-peptide noncovalent complexes formation.J Am Soc Mass Spectrom. 2008 Oct;19(10):1535-41. doi: 10.1016/j.jasms.2008.06.023. Epub 2008 Jul 3. J Am Soc Mass Spectrom. 2008. PMID: 18657435 Free PMC article.
-
Functional Testing and Localization of Tyrosine Sulfation in a Trispecific Antibody.J Am Soc Mass Spectrom. 2025 May 7;36(5):952-960. doi: 10.1021/jasms.4c00432. Epub 2025 Mar 25. J Am Soc Mass Spectrom. 2025. PMID: 40132040
-
Tyrosine sulfation and the secretory pathway.Annu Rev Physiol. 1988;50:363-76. doi: 10.1146/annurev.ph.50.030188.002051. Annu Rev Physiol. 1988. PMID: 3288098 Review.
-
Protein tyrosine sulfation, 1993--an update.Chem Biol Interact. 1994 Jun;92(1-3):257-71. doi: 10.1016/0009-2797(94)90068-x. Chem Biol Interact. 1994. PMID: 8033259 Review.
Cited by
-
Negative Ion Mode Collision-Induced Dissociation for Analysis of Protein Arginine Methylation.J Am Soc Mass Spectrom. 2019 Jul;30(7):1229-1241. doi: 10.1007/s13361-019-02176-9. Epub 2019 Mar 26. J Am Soc Mass Spectrom. 2019. PMID: 30915654 Free PMC article.
-
Modulation of Phosphopeptide Fragmentation via Dual Spray Ion/Ion Reactions Using a Sulfonate-Incorporating Reagent.Anal Chem. 2016 Aug 16;88(16):8158-65. doi: 10.1021/acs.analchem.6b01901. Epub 2016 Aug 8. Anal Chem. 2016. PMID: 27467576 Free PMC article.
-
Tyrosine O-sulfation proteoforms affect HIV-1 monoclonal antibody potency.Sci Rep. 2022 May 19;12(1):8433. doi: 10.1038/s41598-022-12423-x. Sci Rep. 2022. PMID: 35589938 Free PMC article.
-
Discrimination between peptide O-sulfo- and O-phosphotyrosine residues by negative ion mode electrospray tandem mass spectrometry.J Am Soc Mass Spectrom. 2011 Dec;22(12):2256-68. doi: 10.1007/s13361-011-0248-z. Epub 2011 Sep 27. J Am Soc Mass Spectrom. 2011. PMID: 21952787
-
Substrate binding plasticity revealed by Cryo-EM structures of SLC26A2.Nat Commun. 2024 Apr 29;15(1):3616. doi: 10.1038/s41467-024-48028-3. Nat Commun. 2024. PMID: 38684689 Free PMC article.
References
-
- Bettelheim FR. Tyrosine-O-sulfate in a peptide from fibrinogen. J Am Chem Soc. 1954;76:2838–2839.
-
- Hille A, Rosa P, Huttner WB. Tyrosine sulfation: a post-translational modification of proteins destined for secretion? FEBS Lett. 1984;177:129–134. - PubMed
-
- Huttner WB. Tyrosine sulfation and the secretory pathway. Annu Rev Physiol. 1988;50:363–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

