Prioritizing children's medicines for research: a pharmaco-epidemiological study of antiepileptic drugs
- PMID: 17257162
- PMCID: PMC2000594
- DOI: 10.1111/j.1365-2125.2006.02842.x
Prioritizing children's medicines for research: a pharmaco-epidemiological study of antiepileptic drugs
Abstract
Aims: To investigate the prescribing epidemiology, in UK primary care, of newer antiepileptic drugs (AEDs) compared with conventional AEDs and to identify AEDs for further research in response to the European Medicines Agency report on epilepsy.
Methods: Subjects aged 0-18 years, from the UK General Practice Research Database, who were prescribed an AED between 1993 and 2005. Prescribing prevalence and incidence, stratified by age and AED, were calculated.
Results: A total of 7721 subjects were included and 70% were prescribed one drug. Overall prescribing prevalence for all AEDs had increased by 19%. The prevalence (95% confidence interval) of newer AED prescribing had increased fivefold from 0.67 (0.58, 0.76) to 3.20 (3.03, 3.37) per 1000 person-years. Conversely, the prevalence of conventional AEDs had declined by 17% from 6.63 (6.34, 6.92) per 1000 person-years to 5.51 (5.28, 5.73). Lamotrigine had 65% of newer AED prescriptions and was the most prescribed newer drug for both the 2-11 years and 12-18 years age groups with prevalences of 1.47 and 2.55 per 1000 person-years, respectively.
Conclusions: There is a rapid increase in newer AED prescribing to children and adolescents in UK primary care, while prescribing of conventional AEDs is declining. Since 1997, the prevalence of vigabatrin has fallen, coinciding with the UK safety warnings on visual field defects. The uptake of lamotrigine, topiramate and levetiracetam is rapid and as the safety of these drugs has not been established, they should be prioritized for further research. Following concerns with vigabatrin, long-term safety surveillance of all newer AEDs is strongly recommended.
Figures
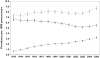
 ), new AEDs
), new AEDs  ), all AEDs (
), all AEDs ( )
)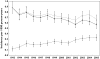
 ), conventional AEDs (
), conventional AEDs ( ), new AEDs (
), new AEDs ( )
)
 ), gabapentin (
), gabapentin ( ), lamotrigine (
), lamotrigine ( ), oxcarbazepine (
), oxcarbazepine ( ), tiagabine (
), tiagabine ( ), topiramate (
), topiramate ( ), vigabatrin (
), vigabatrin ( )
)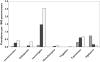
 ), 2–11 years (▪), 12–18 years (□)
), 2–11 years (▪), 12–18 years (□)References
-
- US Food and Drug Administration. The Paediatric Research Equity Act. 22 may 2006. Public Law 108–155. Available at http://www.fda.gov/cder/pediatric/S-650-PREA.pdfLast accessed.
-
- US Department of Health and Human Services, National Institutes of Health. 22 may 2006. Available at http://www.nih.gov/ Last accessed.
-
- European Commission. Better Medicines for Children: Proposed Regulatory Actions on Paediatric Medicinal Products. 17 may 2006. Available at http://pharmacos.eudra.org/F2/pharmacos/docs/Doc2002/feb/cd_pediatrics_e... Last accessed.
-
- The European Agency for the Evaluation of Medicinal Products and Human Medicines Evaluation Unit. Report of the Experts Round Table on the Difficulties Related to the Use of New Medicinal Products in Children Held on 18 December 1997. 17 may 2006. Available at http://www.emea.eu.int/pdfs/human/regaffair/2716498en.pdf Last accessed.
-
- European Medicines Agency. 17 may 2006. Available at http://www.emea.eu.int/htms/human/peg/pegassessment.htm# Last accessed.

