Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models
- PMID: 17257424
- PMCID: PMC1851398
- DOI: 10.1186/bcr1645
Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models
Abstract
Introduction: The experiments reported here address the question of whether a short-term hormone treatment can prevent mammary tumorigenesis in two different genetically engineered mouse models.
Methods: Two mouse models, the p53-null mammary epithelial transplant and the c-neu mouse, were exposed to estrogen and progesterone for 2 and 3 weeks, respectively, and followed for development of mammary tumors.
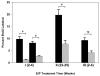
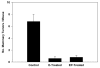
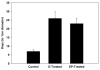
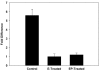
Results: In the p53-null mammary transplant model, a 2-week exposure to estrogen and progesterone during the immediate post-pubertal stage (2 to 4 weeks after transplantation) of mammary development decreased mammary tumorigenesis by 70 to 88%. At 45 weeks after transplantation, analysis of whole mounts of the mammary outgrowths demonstrated the presence of premalignant hyperplasias in both control and hormone-treated glands, indicating that the hormone treatment strongly affects the rate of premalignant progression. One possible mechanism for the decrease in mammary tumorigenesis may be an altered proliferation activity as the bromodeoxyuridine labeling index was decreased by 85% in the mammary glands of hormone-treated mice. The same short-term exposure administered to mature mice at a time of premalignant development also decreased mammary tumorigenesis by 60%. A role for stroma and/or systemic mediated changes induced by the short-term hormone (estrogen/progesterone) treatment was demonstrated by an experiment in which the p53-null mammary epithelial cells were transplanted into the cleared mammary fat pads of previously treated mice. In such mice, the tumor-producing capabilities of the mammary cells were also decreased by 60% compared with the same cells transplanted into unexposed mice. In the second set of experiments using the activated Her-2/neu transgenic mouse model, short-term estradiol or estradiol plus progesterone treatment decreased mammary tumor incidence by 67% and 63%, and tumor multiplicity by 91% and 88%, respectively. The growth rate of tumors arising in the hormone-treated activated Her-2/neu mice was significantly lower than tumors arising in non-hormone treated mice.
Conclusion: Because these experiments were performed in model systems that mimic many essential elements of human breast cancer, the results strengthen the rationale for translating this prevention strategy to humans at high risk for developing breast cancer.
Figures






Comment in
-
Roles for estrogen and progesterone in breast cancer prevention.Breast Cancer Res. 2007;9(2):102. doi: 10.1186/bcr1659. Breast Cancer Res. 2007. PMID: 17381827 Free PMC article.
References
-
- Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, ATAC Trialists' Group Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(05)74803-0. - DOI - PubMed
-
- Willett WC, Rockhill B, Hawkinson SE, Hunter D, Colditz GA. Nongenetic factors in the causation of breast cancer. In: Harris JA, Lippman ME, Morrow M, Osborne CK, editor. Diseases of the Breast. 3. Philadelphia: Lippincott, Williams & Wilkins; 2004. pp. 223–276.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

