Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis
- PMID: 17257598
- PMCID: PMC1861834
- DOI: 10.1016/j.febslet.2007.01.021
Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis
Abstract
Myosin 1E is one of two "long-tailed" human Class I myosins that contain an SH3 domain within the tail region. SH3 domains of yeast and amoeboid myosins I interact with activators of the Arp2/3 complex, an important regulator of actin polymerization. No binding partners for the SH3 domains of myosins I have been identified in higher eukaryotes. In the current study, we show that two proteins with prominent functions in endocytosis, synaptojanin-1 and dynamin, bind to the SH3 domain of human Myo1E. Myosin 1E co-localizes with clathrin- and dynamin-containing puncta at the plasma membrane and this co-localization requires an intact SH3 domain. Expression of Myo1E tail, which acts in a dominant-negative manner, inhibits endocytosis of transferrin. Our findings suggest that myosin 1E may contribute to receptor-mediated endocytosis.
Figures

The domain structure of Myo1E and the tail constructs used to investigate Myo1E localization. Myo1E heavy chain is composed of an N-terminal motor domain, a neck domain with a single IQ light chain binding motif, and a tail domain. Myo1E tail consists of a positively charged TH1 domain, a proline-rich TH2, and an SH3 (TH3) domain [2].
Myo1E construct used as a bait for the yeast two-hybrid screening consists of TH2 and SH3 domain. Synaptojanin-1 (SJ-1) contains two phosphatase domains (Sac1-homology and inositol-5-phopshatase) and a C-terminal PRD. Synaptojanin-1 fragment isolated in the yeast two hybrid screen includes a portion of the inositol 5-phosphatase domain and the PRD.
Interaction of Myo1E with SJ-1 in a yeast two-hybrid assay. Yeast clone containing both Myo1E bait construct and SJ-1 construct exhibits signs of a positive interaction: the ability to grow on the nutrient-deficient medium and development of blue color due to ß-galactosidase reaction. Negative controls (Myo1E bait plus empty vector, SJ-1 construct plus empty vector, SJ-1 construct plus non-interacting protein (Lamin C)) do not grow on the nutrient-deficient medium.

Myo1E binding proteins from rat brain extracts. Immunoblot analysis indicates that both GST-tagged Myo1E SH3 and endophilin-SH3 but not GST alone bind synaptojanin-1 (SJ-1), dynamin-1, and dynamin-2 (Dyn-1,2). A Coomassie stained gel showing the amount of GST fusion proteins used for pulldowns is shown in the lower panel (GST fusions).
Binding of purified synaptojanin-1 and dynamin to Myo1E SH3 domain. SDS-PAGE of glutathione-bead pellet (P) and supernatant (S) fractions followed by Coomassie staining indicates that GST-tagged Myo1E SH3 but not mutant SH3 or GST bind purified synaptojanin-1 and dynamin. Note that mobility shift of dynamin band in lane 3 (SH3 pellet) is a gel artifact caused by the difference in total protein load between the lanes. Right-hand panels show Coomassie stained gels of the purified synaptojanin-1 and dynamin preparations used.
Binding of synaptojanin-1, dynamin-1, and dynamin-2 expressed in cultured cells to Myo1E SH3. Cos-1 cells transfected with EGFP-tagged dynamin-1 or myc-tagged synaptojanin-1 (partial clone isolated from the kidney cDNA library), HeLa cells transfected with untagged dynamin-2 or non-transfected HeLa cells were lysed and cell lysates were incubated with GST-tagged proteins as indicated. Immunoblots indicated that overexpressed synaptojanin-1 and dynamin-1 and -2 as well as endogenous dynamin-2 (End. Dyn-2) from HeLa lysate bound to GST-SH3 but not pure GST or mutant SH3 (SH3WK). Lower panels show Coomassie stained gels of the GST fusions used.
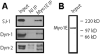
Immunoblot analysis indicated that synaptojanin-1, dynamin-1 and -2 were present in Myo1E immunoprecipitates but not in non-immune immunoprecipitates.
Anti-Myo1E antibody used for immunoprecipitation recognized a single band in the synapse-enriched rat brain preparation. Positions of molecular weight markers are shown on the right.

Swiss3T3 fibroblasts stably expressing DsRed-clathrin (Cla) were transfected with EGFP-tagged Myo1E, Myo1E tail containing all three tail homology regions (TH1, TH2, SH3), mutant tail (tailWK) or partial tail constructs (TH1 only, TH2+SH3 (TH2+3), SH3 only) and imaged using TIRF microscopy. Vesicles labeled with both green and red fluorescent proteins are marked by arrows. Scale bar - 1 μm.
Frequency of colocalization of various Myo1E constructs with clathrin-positive puncta. Percentage of clathrin-coated vesicles (CCVs) labeled with EGFP-tagged Myo1E constructs was determined as described in Materials and Methods. Numbers shown represent average percentages+/-SD for 5 cells for Myo1E, tailWK, and SH3 constructs, 7 cells for TH2+3 and TH1, and 10 cells for Myo1E tail. Average percent colocalization for each construct was compared with the average percent colocalization for Myo1E tail using t-test. * - P< 0.05, ** - P<0.005, *** - P<0.001.
Colocalization of EGFP-Myo1E and mRFP-dynamin-1 in Swiss3T3 cells by TIRF microscopy. Arrows indicate fluorescent puncta that contain both EGFP-Myo1E and dynamin. Scale bar - 1 um.

Swiss3T3 fibroblasts stably expressing DsRed-clathrin (Cla) were transfected with EGFP-tagged Myo1E, Myo1E tail containing all three tail homology regions (TH1, TH2, SH3), mutant tail (tailWK) or partial tail constructs (TH1 only, TH2+SH3 (TH2+3), SH3 only) and imaged using TIRF microscopy. Vesicles labeled with both green and red fluorescent proteins are marked by arrows. Scale bar - 1 μm.
Frequency of colocalization of various Myo1E constructs with clathrin-positive puncta. Percentage of clathrin-coated vesicles (CCVs) labeled with EGFP-tagged Myo1E constructs was determined as described in Materials and Methods. Numbers shown represent average percentages+/-SD for 5 cells for Myo1E, tailWK, and SH3 constructs, 7 cells for TH2+3 and TH1, and 10 cells for Myo1E tail. Average percent colocalization for each construct was compared with the average percent colocalization for Myo1E tail using t-test. * - P< 0.05, ** - P<0.005, *** - P<0.001.
Colocalization of EGFP-Myo1E and mRFP-dynamin-1 in Swiss3T3 cells by TIRF microscopy. Arrows indicate fluorescent puncta that contain both EGFP-Myo1E and dynamin. Scale bar - 1 um.


Transferrin (Tfn) uptake in EGFP-Myo1E tail-transfected HeLa cells. Cells expressing Myo1E tail (arrows) did not exhibit punctate transferrin labeling. Scale bar - 20 μm.
Quantitative analysis of transferrin endocytosis in HeLa cells. Cells transfected with various DNA constructs were allowed to take up fluorescent transferrin and processed as in A. Percent of transfected cells exhibiting punctate transferrin labeling was determined from three independent experiments (N>/=100 cells for each experiment, error bars=SD).
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

