Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC
- PMID: 17258081
- PMCID: PMC1847625
- DOI: 10.1016/j.exphem.2006.09.017
Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC
Abstract
Objective: Faster engraftment of G-CSF-mobilized peripheral blood (MPB) transplants compared to steady-state bone marrow (ssBM) is well documented and clinically relevant. A number of different factors likely contribute to this outcome. In the present study we explored whether independent of cell number there are intrinsic differences in the efficiency of progenitor cell homing to marrow between MPB and ssBM.
Methods: Mobilization was achieved by continuous infusion of G-CSF alone or in combination with other mobilizing agents. In vivo homing assays, in vitro migration assays, gene expression analysis, and flow cytometry were utilized to compare homing-related in vivo and in vitro properties of MPB and ssBM HPC.
Results: Marrow homing of murine MPB HPC, generated by different mobilizing schemes, was reproducibly significantly superior to that of ssBM, in lethally irradiated as well as in nonirradiated hosts. This phenotype was independent of MMP9, selectins, and beta2- and alpha4-integrins. Superior homing was also observed for human MPB HPC transplanted into NOD/SCIDbeta2microglobulin(-/-) recipients. Inhibition of HPC migration abrogated the homing advantage of MPB but did not affect homing of ssBM HPC, whereas enhancement of motility by CD26 inhibition improved marrow homing only of ssBM HPC. Enhanced SDF-1-dependent chemotaxis and low CD26 expression on MPB HPC were identified as potential contributing factors. Significant contributions of the putative alternative SDF-1 receptor, RDC1, were unlikely based on gene expression data.
Conclusion: The data suggest increased motility as a converging endpoint of complex changes seen in MPB HPC which is likely responsible for their favorable homing.
Figures

 , ssBM
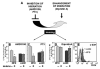
, ssBM  ). D: Forty-five% more efficient mean marrow homing of MPB CFU-C compared to ssBM CFU-C was observed 18 hours after transplantation of lethally irradiated isogeneic recipients (p<0.001). Each dot represents homing in one individual animal; bold line and error bars represent mean±SEM for % of injected CFU-C homed to marrow. E: Marrow homing of human CD34+ cells from ssBM or MPB was tested in a xenotransplantation model. Eighteen hours after transplantation, the mean recovery of MPB CFU-C from recipient marrow was 3-fold increased compared to ssBM (*p<0.001). Each dot represents homing in one individual animal; bold line and error bars represent mean±SEM for % of injected CFU-C homed to marrow. These data indicate that the greater marrow homing ability of MPB CFU-C is intrinsic to a population enriched for HPC, and that it applies to human as well as to mouse CFU-C.
). D: Forty-five% more efficient mean marrow homing of MPB CFU-C compared to ssBM CFU-C was observed 18 hours after transplantation of lethally irradiated isogeneic recipients (p<0.001). Each dot represents homing in one individual animal; bold line and error bars represent mean±SEM for % of injected CFU-C homed to marrow. E: Marrow homing of human CD34+ cells from ssBM or MPB was tested in a xenotransplantation model. Eighteen hours after transplantation, the mean recovery of MPB CFU-C from recipient marrow was 3-fold increased compared to ssBM (*p<0.001). Each dot represents homing in one individual animal; bold line and error bars represent mean±SEM for % of injected CFU-C homed to marrow. These data indicate that the greater marrow homing ability of MPB CFU-C is intrinsic to a population enriched for HPC, and that it applies to human as well as to mouse CFU-C.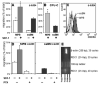
 , ssBM
, ssBM  , solid: - inhibitor, hatched: + inhibitor; * indicates significant difference (p<0.05) compared to samples from the same source that were not treated with the inhibitor). The FACS histogram depicts expression of CD26 on c-kit+ cells (Isotype control lightly shaded, MPB black line, ssBM solid grey; cell counts on Y-axis, fluorescence intensity on X-axis).
, solid: - inhibitor, hatched: + inhibitor; * indicates significant difference (p<0.05) compared to samples from the same source that were not treated with the inhibitor). The FACS histogram depicts expression of CD26 on c-kit+ cells (Isotype control lightly shaded, MPB black line, ssBM solid grey; cell counts on Y-axis, fluorescence intensity on X-axis).
 , ssBM
, ssBM  ).
).Similar articles
-
A novel anti-inflammatory function of human galectin-1: inhibition of hematopoietic progenitor cell mobilization.Exp Hematol. 2007 Feb;35(2):305-13. doi: 10.1016/j.exphem.2006.09.015. Exp Hematol. 2007. PMID: 17258079
-
Treatment of circulating CD34(+) cells with SDF-1alpha or anti-CXCR4 antibody enhances migration and NOD/SCID repopulating potential.Exp Hematol. 2002 Sep;30(9):1061-9. doi: 10.1016/s0301-472x(02)00880-9. Exp Hematol. 2002. PMID: 12225798
-
[Differential study on the in vivo homing potential of human hematopoietic stem/progenitor cells from different sources in xenotransplanted NOD/SCID mouse model].Zhonghua Xue Ye Xue Za Zhi. 2007 Jun;28(6):391-5. Zhonghua Xue Ye Xue Za Zhi. 2007. PMID: 17939405 Chinese.
-
Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions.Ann N Y Acad Sci. 2001 Jun;938:83-95. doi: 10.1111/j.1749-6632.2001.tb03577.x. Ann N Y Acad Sci. 2001. PMID: 11458529 Review.
-
Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties.Exp Hematol. 2006 Aug;34(8):1010-20. doi: 10.1016/j.exphem.2006.04.004. Exp Hematol. 2006. PMID: 16863907 Review.
Cited by
-
The role of dipeptidyl peptidase 4 in hematopoiesis and transplantation.Curr Opin Hematol. 2013 Jul;20(4):314-9. doi: 10.1097/MOH.0b013e32836125ac. Curr Opin Hematol. 2013. PMID: 23594692 Free PMC article. Review.
-
Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab.Blood. 2008 Apr 1;111(7):3439-41. doi: 10.1182/blood-2007-09-112052. Epub 2008 Jan 14. Blood. 2008. PMID: 18195093 Free PMC article.
-
The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes.Stem Cell Rev Rep. 2011 Sep;7(3):590-607. doi: 10.1007/s12015-010-9212-8. Stem Cell Rev Rep. 2011. PMID: 21140298 Free PMC article. Review.
-
Chemokine-mobilized adult stem cells; defining a better hematopoietic graft.Leukemia. 2008 Mar;22(3):466-73. doi: 10.1038/sj.leu.2405021. Epub 2007 Nov 1. Leukemia. 2008. PMID: 17972941 Free PMC article. Review.
-
The novel CXCR4 antagonist POL5551 mobilizes hematopoietic stem and progenitor cells with greater efficiency than Plerixafor.Leukemia. 2013 Dec;27(12):2322-31. doi: 10.1038/leu.2013.266. Epub 2013 Sep 12. Leukemia. 2013. PMID: 24072044 Free PMC article.
References
-
- Chao NJ, Schriber JR, Grimes K, et al. Granulocyte colony-stimulating factor “mobilized” peripheral blood progenitor cells accelerate granulocyte and platelet recovery after high-dose chemotherapy. Blood. 1993;81:2031–2035. - PubMed
-
- Ringden O, Remberger M, Runde V, et al. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood. 1999;94:455–464. - PubMed
-
- Schmitz N, Linch DC, Dreger P, et al. Randomised trial of filgrastimmobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–357. - PubMed
-
- Mertelsmann R, Herrmann F, Hecht T, Schulz G. Hematopoietic growth factors in bone marrow transplantation. Bone Marrow Transplant. 1990;6:73–77. - PubMed
-
- Hassan HT, Zeller W, Stockschlader M, Kruger W, Hoffknecht MM, Zander AR. Comparison between bone marrow and G-CSF-mobilized peripheral blood allografts undergoing clinical scale CD34+ cell selection. Stem Cells. 1996;14:419–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

