Cardiac allograft vasculopathy: real or a normal morphologic variant?
- PMID: 17258151
- PMCID: PMC1802125
- DOI: 10.1016/j.healun.2006.11.012
Cardiac allograft vasculopathy: real or a normal morphologic variant?
Abstract
Background: Naive coronary vessels may appear to have intimal thickening histologically characteristic of cardiac allograft vasculopathy (CAV). This study appraises the experimental and clinical impact of this observation.
Methods: Tissue sections from 12 naive hearts of miniature swine, 13 native porcine hearts of recipients of heterotopic cardiac allografts, 3 native human hearts and 3 human hearts with CAV were compared with light microscopy and morphometric analysis. Results were also compared with morphometric data previously gathered from 3 grafts in a standard experimental model of CAV (rejectors) and 3 grafts harvested from swine rendered tolerant to their donor hearts (chimeras).
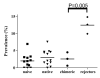
Results: In the naive and native porcine hearts, the prevalence of CAV "mimics" was 0% to 6.94% (mean +/- SD: 1.99 +/- 1.97%) and 0% to 7.57% (2.97 +/- 2.20%), respectively (p = 0.12). The prevalence of CAV in the grafts of porcine rejectors and chimeras was 9.9% to 14.8% (12.4 +/- 2.5%) and 0.6% to 4.5% (2.6 +/- 2.0%), respectively (p < 0.05). CAV in the chimeras was similar in prevalence to that of the naive and native hearts. In native human hearts and human grafts, the prevalence was 1.86% to 2.00% (1.95 +/- 0.08%) and 9.09% to 17.50% (12.80 +/- 4.29%), respectively (p = 0.01).
Conclusions: Smooth muscle bundles inside the internal elastic laminae are similarly prevalent in human and porcine coronary vasculature. Their histologic similarity to intimal thickening of CAV could lead to an inaccurate distinction between graft tolerance and CAV in both clinical and experimental studies of heart transplantation.
Figures





References
-
- Shirwan H. Chronic allograft rejection. Do the TH2 cells preferentially induced by indirect alloantigen recognition play a dominant role? Transplantation. 1999;68:715–26. - PubMed
-
- Yamani MH, Yousufuddin M, Starling RC, et al. Does acute cellular rejection correlate with cardiac allograft vasculopathy? J Heart Lung Transplant. 2004;23:272–76. - PubMed
-
- Andersen HØ. Heart allograft vascular disease, an obliterative vascular disease in transplanted hearts. Atherosclerosis. 1999;142:243–63. - PubMed
-
- Thomson JG. Production of severe atheroma in a transplanted human heart. Lancet. 1969;2:1088–92. - PubMed
-
- Valantine H. Cardiac allograft vasculopathy after heart transplantation risk factors and management. J heart Lung Transplant. 2004;23:S187–93. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

