N-thiolated beta-lactams: Studies on the mode of action and identification of a primary cellular target in Staphylococcus aureus
- PMID: 17258460
- PMCID: PMC1850389
- DOI: 10.1016/j.bmc.2006.12.027
N-thiolated beta-lactams: Studies on the mode of action and identification of a primary cellular target in Staphylococcus aureus
Abstract
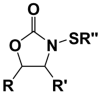
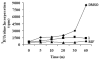
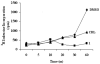
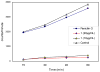
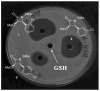
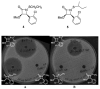
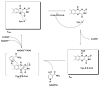
This study focuses on the mechanism of action of N-alkylthio beta-lactams, a new family of antibacterial compounds that show promising activity against Staphylococcus and Bacillus microbes. Previous investigations have determined that these compounds are highly selective towards these bacteria, and possess completely unprecedented structure-activity profiles for a beta-lactam antibiotic. Unlike penicillin, which inhibits cell wall crosslinking proteins and affords a broad spectrum of bacteriocidal activity, these N-thiolated lactams are bacteriostatic in their behavior and act through a different mechanistic mode. Our current findings indicate that the compounds react rapidly within the bacterial cell with coenzyme A (CoA) through in vivo transfer of the N-thio group to produce an alkyl-CoA mixed disulfide species, which then interferes with fatty acid biosynthesis. Our studies on coenzyme A disulfide reductase show that the CoA thiol-redox buffer is not perturbed by these compounds; however, the lactams appear to act as prodrugs. The experimental evidence that these beta-lactams inhibit fatty acid biosynthesis in bacteria, and the elucidation of coenzyme A as a primary cellular target, offers opportunities for the discovery of other small organic compounds that can be developed as therapeutics for MRSA and anthrax infections.
Figures
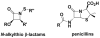



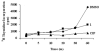
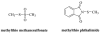

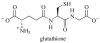

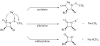
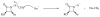
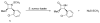

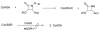
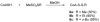
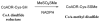
References
-
- Turos E, Konaklieva MI, Ren RXF, Shi H, Gonzalez J, Dickey S, Lim D. Tetrahedron. 2000;56:8193. 2000.
-
- Turos E, Long TE, Konaklieva MI, Coates C, Shim JY, Dickey S, Lim DV, Cannons A. Bioorg Med Chem Lett. 2002;12:2229. - PubMed
-
- Coates C, Long TE, Turos E, Dickey S, Lim DV. Bioorg Med Chem Lett. 2003;11:193. - PubMed
-
- Long TE, Turos E, Konaklieva MI, Blum AL, Amry A, Baker EA, Suwandi LS, McCain MD, Rahman MF, Dickey S, Lim DV. Bioorg Med Chem. 2003;11:1859. - PubMed
-
- Turos E, Coates C, Shim JY, Wang Y, Roettgers JM, Long TE, Reddy GSK, Ortiz A, Culbreath M, Dickey S, Lim DV, Alonso E, Gonzalez J. Bioorg Med Chem. 2005;13:6289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

