Spatio-temporal localization of membrane lipid rafts in mouse oocytes and cleaving preimplantation embryos
- PMID: 17258703
- PMCID: PMC1861839
- DOI: 10.1016/j.ydbio.2006.12.009
Spatio-temporal localization of membrane lipid rafts in mouse oocytes and cleaving preimplantation embryos
Abstract
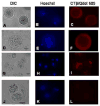
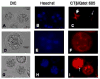
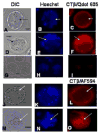
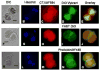
We report for the first time the detection of membrane lipid rafts in mouse oocytes and cleaving preimplantation embryos. Cholera toxin beta (CTbeta), which binds to the raft-enriched ganglioside GM1, was selected to label rafts. In a novel application a Qdot reagent was used to detect CTbeta labeling. This is the first reported use of nanocrystals in mammalian embryo imaging. Comparative membrane labeling with CTbeta and lipophilic membrane dyes containing saturated or unsaturated aliphatic tails showed that the detection of GM1 in mouse oocytes and embryo membranes was consistent with the identification of cholesterol- and sphingolipid-enriched rafts in the cell membrane. Distribution of the GM1 was compared with the known distribution of non-raft membrane components, and disruption of membrane rafts with detergents confirmed the cholesterol dependence of GM1 on lipid raft labeling. Complementary functional studies showed that cholesterol depletion using methyl-beta-cyclodextrin inhibited preimplantation development in culture. Our results show that the membranes of the mouse oocyte and zygote are rich in lipid rafts, with heterogeneous and stage-dependent distribution. In dividing embryos, the rafts were clearly associated with the cleavage furrow. At the morula stage, rafts were also apically enriched in each blastomere. In blastocysts, rafts were detectable in the trophectoderm layer, but could not be detected in the inner cell mass without prior fixation and permeabilization of the embryo. Lipid rafts and their associated proteins are, therefore, spatio-temporally positioned to a play a critical role in preimplantation developmental events.
Figures



References
-
- Albertson R, et al. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. - PubMed
-
- Allworth A, Ziomek CA. Filipin-labelled complexes are polarized in their distribution in the cytoplasm of meiotically mature mouse eggs. Gamete Res. 1988;20:475–89. - PubMed
-
- Belton RJ, Jr, et al. Isolation and characterization of sea urchin egg lipid rafts and their possible function during fertilization. Mol Reprod Dev. 2001;59:294–305. - PubMed
-
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

