Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation
- PMID: 17259073
- PMCID: PMC1805469
- DOI: 10.1016/j.prostaglandins.2006.09.009
Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation
Abstract
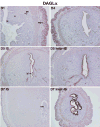
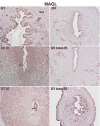
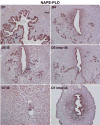
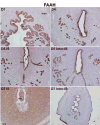
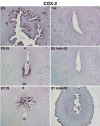
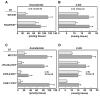
Preimplantation embryo development to the blastocyst stage and uterine differentiation to the receptive state are prerequisites for embryo implantation. Burgeoning evidence suggests that endocannabinoid signaling is critical to early pregnancy events. Anandamide (N-arachidonoylethanolamine) and 2-AG (2-arachidonoylglycerol) are two major endocannabinoids that bind to and activate G-protein coupled cannabinoid receptors CB1 and CB2. We have previously shown that a physiological tone of anandamide is critical to preimplantation events in mice, since either silencing or amplification of anandamide signaling causes retarded development and oviductal retention of embryos via CB1, leading to deferred implantation and compromised pregnancy outcome. Whether 2-AG, which also influences many biological functions, has any effects on early pregnancy remains unknown. Furthermore, mechanisms by which differential uterine endocannabinoid gradients are established under changing pregnancy state is not clearly understood. We show here that 2-AG is present at levels one order of magnitude higher than those of anandamide in the mouse uterus, but with similar patterns as anandamide, i.e. lower levels at implantation sites and higher at interimplantation sites. We also provide evidence that region- and stage-specific uterine expression of N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH), and sn-1-diacylglycerol (DAG) lipase alpha (DAGLalpha) and monoacylglycerol lipase (MAGL) for synthesis and hydrolysis of anandamide and 2-AG, respectively, creates endocannabinoid gradients conducive to implantation. Our genetic evidence suggests that FAAH is the major degrading enzyme for anandamide, whereas COX-2, MAGL and to some extent COX-1 participate in metabolizing 2-AG in the pregnant uterus. The results suggest that aberrant functioning of these pathways impacting uterine anandamide and/or 2-AG levels would compromise pregnancy outcome.
Figures

References
-
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. - PubMed
-
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. - PubMed
-
- Paria BC, Dey SK. Ligand-receptor signaling with endocannabinoids in preimplantation embryo development and implantation. Chem Phys Lipids. 2000;108:211–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

