Spectroscopic observation of RNA chaperone activities of Hfq in post-transcriptional regulation by a small non-coding RNA
- PMID: 17259214
- PMCID: PMC1807976
- DOI: 10.1093/nar/gkl1124
Spectroscopic observation of RNA chaperone activities of Hfq in post-transcriptional regulation by a small non-coding RNA
Abstract
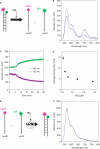
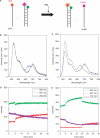
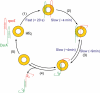
Hfq protein is vital for the function of many non-coding small (s)RNAs in bacteria but the mechanism by which Hfq facilitates the function of sRNA is still debated. We developed a fluorescence resonance energy transfer assay to probe how Hfq modulates the interaction between a sRNA, DsrA, and its regulatory target mRNA, rpoS. The relevant RNA fragments were labelled so that changes in intra- and intermolecular RNA structures can be monitored in real time. Our data show that Hfq promotes the strand exchange reaction in which the internal structure of rpoS is replaced by pairing with DsrA such that the Shine-Dalgarno sequence of the mRNA becomes exposed. Hfq appears to carry out strand exchange by inducing rapid association of DsrA and a premelted rpoS and by aiding in the slow disruption of the rpoS secondary structure. Unexpectedly, Hfq also disrupts a preformed complex between rpoS and DsrA. While it may not be a frequent event in vivo, this melting activity may have implications in the reversal of sRNA-based regulation. Overall, our data suggests that Hfq not only promotes strand exchange by binding rapidly to both DsrA and rpoS but also possesses RNA chaperoning properties that facilitates dynamic RNA-RNA interactions.
Figures



References
-
- Franze de Fernandez MT, Hayward WS, August JT. Bacterial proteins required for replication of phage Qβ ribonucleic acid. J. Biol. Chem. 1972;247:824–821. - PubMed
-
- Rasmussen AA, Eriksen M, Gilany K, Udesen C, Franch T, Petersen C, Valentin-Hansen P. Regulation of ompA mRNA stability: the role of a small regulatory RNA in growth phase-dependent control. Mol. Microbiol. 2005;58:1421–1429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

