CRYPTOCHROME2 in vascular bundles regulates flowering in Arabidopsis
- PMID: 17259260
- PMCID: PMC1820968
- DOI: 10.1105/tpc.106.048157
CRYPTOCHROME2 in vascular bundles regulates flowering in Arabidopsis
Abstract
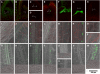
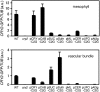
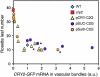
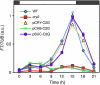
Plants make full use of light signals to determine the timing of flowering. In Arabidopsis thaliana, a blue/UV-A photoreceptor, CRYPTOCHROME 2 (cry2), and a red/far-red photoreceptor, PHYTOCHROME B (phyB), are two major photoreceptors that control flowering. The light stimuli for the regulation of flowering are perceived by leaves. We have recently shown that phyB expression in mesophyll but not in vascular bundles suppresses the expression of a key flowering regulator, FLOWERING LOCUS T (FT), in vascular bundles. In this study, we asked where in the leaf cry2 perceives light stimuli to regulate flowering. To answer this question, we established transgenic Arabidopsis lines in which the cry2-green fluorescent protein (GFP) fusion was expressed under the control of organ/tissue-specific promoters in a cry2-deficient mutant background. Analysis of these lines revealed that expression of cry2-GFP in vascular bundles, but not in epidermis or mesophyll, rescued the late flowering phenotype. We further confirmed that cry2-GFP expressed in vascular bundles increased FT expression only in vascular bundles. Hence, in striking contrast with phyB, cry2 most likely regulates FT expression in a cell-autonomous manner.
Figures



References
-
- An, H., Roussot, C., Suarez-Lopez, P., Corbesier, L., Vincent, C., Pineiro, M., Hepworth, S., Mouradov, A., Justin, S., Turnbull, C., and Coupland, G. (2004). CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131 3615–3626. - PubMed
-
- Black, M., and Shuttleworth, J.E. (1974). The role of the cotyledons in the photocontrol of hypocotyl extension in Cucumis sativus L. Planta 117 57–66. - PubMed
-
- Briggs, W.R., and Christie, J.M. (2002). Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 7 204–210. - PubMed
-
- Casal, J.J., and Smith, H. (1988). Persistent effects of changes in phytochrome status on internode growth in light-grown mustard: Occurrence, kinetics and locus of perception. Planta 175 214–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

