The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis
- PMID: 17259262
- PMCID: PMC1820950
- DOI: 10.1105/tpc.106.048033
The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis
Abstract
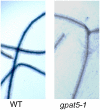
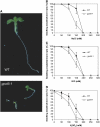
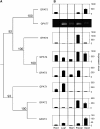
Suberin and cutin are fatty acid- and glycerol-based plant polymers that act as pathogen barriers and function in the control of water and solute transport. However, despite important physiological roles, their biosynthetic pathways, including the acyl transfer reactions, remain hypothetical. We report the characterization of two suberin mutants (gpat5-1 and gpat5-2) of Arabidopsis thaliana GPAT5, encoding a protein with acyl-CoA:glycerol-3-phosphate acyltransferase activity. RT-PCR and beta-glucuronidase-promoter fusion analyses demonstrated GPAT5 expression in seed coat, root, hypocotyl, and anther. The gpat5 plants showed a 50% decrease in aliphatic suberin in young roots and produced seed coats with a severalfold reduction in very long chain dicarboxylic acid and omega-hydroxy fatty acids typical of suberin but no change in the composition or content of membrane or storage glycerolipids or surface waxes. Consistent with their altered suberin, seed coats of gpat5 mutants had a steep increase in permeability to tetrazolium salts compared with wild-type seed coats. Furthermore, the germination rate of gpat5 seeds under high salt was reduced, and gpat5 seedlings had lower tolerance to salt stress. These results provide evidence for a critical role of GPAT5 in polyester biogenesis in seed coats and roots and for the importance of lipid polymer structures in the normal function of these organs.
Figures
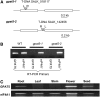
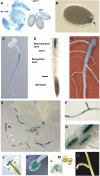
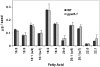
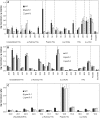




References
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Baum, S.F., Dubrovsky, J.G., and Rost, L.T. (2002). Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. Am. J. Bot. 89 908–920. - PubMed
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. 316 1194–1199. - PubMed
-
- Beeckman, T., De Rycke, R., Viane, R., and Inze, D. (2000). Histological study of seed coat development in Arabidopsis thaliana. J. Plant Res. 113 139–148.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

