Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response
- PMID: 17259265
- PMCID: PMC1820954
- DOI: 10.1105/tpc.106.041426
Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response
Abstract
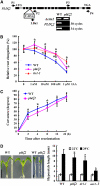
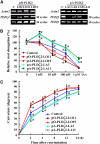
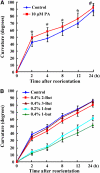
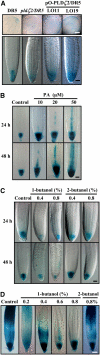
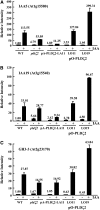
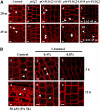
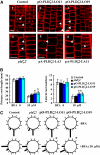
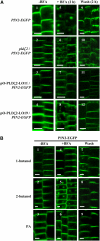
Phospholipase D (PLD) and its product, phosphatidic acid (PA), play key roles in cellular processes, including stress and hormonal responses, vesicle trafficking, and cytoskeletal rearrangements. We isolated and functionally characterized Arabidopsis thaliana PLDzeta2, which is expressed in various tissues and enhanced by auxin. A PLDzeta2-defective mutant, pldzeta2, and transgenic plants deficient in PLDzeta2 were less sensitive to auxin, had reduced root gravitropism, and suppressed auxin-dependent hypocotyl elongation at 29 degrees C, whereas transgenic seedlings overexpressing PLDzeta2 showed opposite phenotypes, suggesting that PLDzeta2 positively mediates auxin responses. Studies on the expression of auxin-responsive genes and observation of the beta-glucuronidase (GUS) expression in crosses between pldzeta2 and lines containing DR5-GUS indicated that PLDzeta2, or PA, stimulated auxin responses. Observations of the membrane-selective dye FM4-64 showed suppressed vesicle trafficking under PLDzeta2 deficiency or by treatment with 1-butanol, a PLD-specific inhibitor. By contrast, vesicle trafficking was enhanced by PA or PLDzeta2 overexpression. Analyses of crosses between pldzeta2 and lines containing PIN-FORMED2 (PIN2)-enhanced green fluorescent protein showed that PLDzeta2 deficiency had no effect on the localization of PIN2 but blocked the inhibition of brefeldin A on PIN2 cycling. These results suggest that PLDzeta2 and PA are required for the normal cycling of PIN2-containing vesicles as well as for function in auxin transport and distribution, and hence auxin responses.
Figures

References
-
- Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wisniewska, J., Moulinier-Anzola, J.C., Sieberer, T., Friml, J., and Luschnig, C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8 249–256. - PubMed
-
- Alfandari, D., and Darribère, T. (1994). A simple PCR method for screening cDNA libraries. PCR Methods Appl. 4 46–49. - PubMed
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Benjamins, R., Quint, A., Weijers, D., Hooykaas, P.J.J., and Offringa, R. (2001). The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

