Contact-induced structure transformation in transmembrane prion propagation
- PMID: 17259269
- PMCID: PMC1831692
- DOI: 10.1529/biophysj.106.098335
Contact-induced structure transformation in transmembrane prion propagation
Abstract
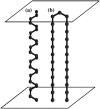
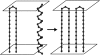
Based on recent experimental evidences of the transmission of prion diseases due to a particular transmembrane form (termed (Ctm)PrP), we propose a theoretical model for the molecular mechanism of such conformational diseases, in which a misfolded (Ctm)PrP induces a similar misfolding of another (Ctm)PrP. Computer simulations are performed to investigate the correlation between folding time and the concentration of misfolded PrP in various processes, including dimerization, trimerization, and cooperative dimerization. By comparing with the experimental correlation curve between incubation time and injected dose of scrapie prions, we conclude that cooperative dimerization may play an important role in the pathological mechanism of prion diseases.
Figures
Similar articles
-
Structure, dynamics, and stability of assemblies of the human prion fragment SNQNNF.Biochem Biophys Res Commun. 2008 Feb 15;366(3):800-6. doi: 10.1016/j.bbrc.2007.12.047. Epub 2007 Dec 17. Biochem Biophys Res Commun. 2008. PMID: 18082625
-
Mass spectroscopic analysis of Sup35NM prion polymerization.Biophys J. 2005 Dec;89(6):4139-48. doi: 10.1529/biophysj.105.063875. Epub 2005 Sep 30. Biophys J. 2005. PMID: 16199512 Free PMC article.
-
Model study of prionlike folding behavior in aggregated proteins.Phys Rev E Stat Nonlin Soft Matter Phys. 2005 Oct;72(4 Pt 1):041912. doi: 10.1103/PhysRevE.72.041912. Epub 2005 Oct 12. Phys Rev E Stat Nonlin Soft Matter Phys. 2005. PMID: 16383425
-
Prion protein oligomer and its neurotoxicity.Acta Biochim Biophys Sin (Shanghai). 2013 Jun;45(6):442-51. doi: 10.1093/abbs/gmt037. Epub 2013 Apr 4. Acta Biochim Biophys Sin (Shanghai). 2013. PMID: 23557632 Review.
-
Modeling of protein misfolding in disease.Methods Mol Biol. 2008;443:297-330. doi: 10.1007/978-1-59745-177-2_16. Methods Mol Biol. 2008. PMID: 18446294 Review.
Cited by
-
Viral channel forming proteins--How to assemble and depolarize lipid membranes in silico.Biochim Biophys Acta. 2016 Jul;1858(7 Pt B):1710-21. doi: 10.1016/j.bbamem.2016.01.018. Epub 2016 Jan 22. Biochim Biophys Acta. 2016. PMID: 26806161 Free PMC article. Review.
References
-
- Weissmann, C. 1999. Molecular genetics of transmissible spongiform encephalopathies. J. Biol. Chem. 274:3–6. - PubMed
-
- Johnson, R. T., and C. J. Gibbs. 1998. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N. Engl. J. Med. 399:1994–2004. - PubMed
-
- Horwich, A. L., and J. S. Weissman. 1997. Deadly conformations—protein misfolding in prion disease. Cell. 89:499–510. - PubMed
-
- Weissmann, C. 2004. The state of the prion. Nat. Rev. Microbiol. 2:861–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

