Mechanism of tension generation in muscle: an analysis of the forward and reverse rate constants
- PMID: 17259275
- PMCID: PMC1831703
- DOI: 10.1529/biophysj.106.101477
Mechanism of tension generation in muscle: an analysis of the forward and reverse rate constants
Abstract
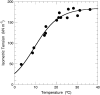
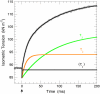
Tension generation in muscle occurs during the attached phase of the ATP-powered cyclic interaction of myosin heads with thin filaments. The transient nature of tension-generating intermediates and the complexity of the mechanochemical cross-bridge cycle have impeded a quantitative description of tension generation. Recent experiments performed under special conditions yielded a sigmoidal dependence of fiber tension on temperature--a unique case that simplifies the system to a two-state transition. We have applied this two-state analysis to kinetic data obtained from biexponential laser temperature-jump tension transients. Here we present the forward and reverse rate constants for de novo tension generation derived from analysis of the kinetics of the fast laser temperature-jump phase tau(2) (equivalent of the length-jump phase 2(slow)). The slow phase tau(3) is temperature-independent indicating coupling to rather than a direct role in, de novo tension generation. Increasing temperature accelerates the forward, and slows the reverse, rate constant for the creation of the tension-generating state. Arrhenius behavior of the forward and anti-Arrhenius behavior of the reverse rate constant is a kinetic signature of multistate multipathway protein-folding reactions. We conclude that locally unfolded tertiary and/or secondary structure of the actomyosin cross-bridge mediates the power stroke.
Figures



Similar articles
-
Kinetic effects of fiber type on the two subcomponents of the Huxley-Simmons phase 2 in muscle.Biophys J. 2003 Jul;85(1):390-401. doi: 10.1016/S0006-3495(03)74483-X. Biophys J. 2003. PMID: 12829493 Free PMC article.
-
Temperature dependence of the force-generating process in single fibres from frog skeletal muscle.J Physiol. 2003 May 15;549(Pt 1):93-106. doi: 10.1113/jphysiol.2002.038703. Epub 2003 Mar 28. J Physiol. 2003. PMID: 12665607 Free PMC article.
-
Force generation and temperature-jump and length-jump tension transients in muscle fibers.Biophys J. 1995 May;68(5):2032-40. doi: 10.1016/S0006-3495(95)80380-2. Biophys J. 1995. PMID: 7612845 Free PMC article.
-
Force generation simplified. Insights from laser temperature-jump experiments on contracting muscle fibers.Adv Exp Med Biol. 1998;453:343-51; discussion 351-2. Adv Exp Med Biol. 1998. PMID: 9889846 Review.
-
What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres?J Muscle Res Cell Motil. 2003;24(2-3):127-38. doi: 10.1023/a:1026093212111. J Muscle Res Cell Motil. 2003. PMID: 14609024 Review.
Cited by
-
Temperature change as a probe of muscle crossbridge kinetics: a review and discussion.Proc Biol Sci. 2009 Aug 7;276(1668):2685-95. doi: 10.1098/rspb.2009.0177. Epub 2009 Apr 8. Proc Biol Sci. 2009. PMID: 19364742 Free PMC article. Review.
-
Mechanistic role of movement and strain sensitivity in muscle contraction.Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6140-5. doi: 10.1073/pnas.0812487106. Epub 2009 Mar 26. Proc Natl Acad Sci U S A. 2009. PMID: 19325123 Free PMC article.
-
Mechanism of force enhancement during and after lengthening of active muscle: a temperature dependence study.J Muscle Res Cell Motil. 2012 Oct;33(5):313-25. doi: 10.1007/s10974-012-9307-8. Epub 2012 Jun 16. J Muscle Res Cell Motil. 2012. PMID: 22706970
-
Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers.Am J Physiol Cell Physiol. 2014 Nov 15;307(10):C939-50. doi: 10.1152/ajpcell.00206.2014. Epub 2014 Sep 3. Am J Physiol Cell Physiol. 2014. PMID: 25186012 Free PMC article.
-
Effects of Hydrostatic-Pressure on Muscle Contraction: A Look Back on Some Experimental Findings.Int J Mol Sci. 2023 Mar 6;24(5):5031. doi: 10.3390/ijms24055031. Int J Mol Sci. 2023. PMID: 36902460 Free PMC article. Review.
References
-
- Davis, J. S. 1998. Force generation simplified. Insights from laser temperature-jump experiments on contracting muscle fibers. Adv. Exp. Med. Biol. 453:343–351 (discussion 351–342). - PubMed
-
- Geeves, M. A., and K. C. Holmes. 1999. Structural mechanism of muscle contraction. Annu. Rev. Biochem. 68:687–728. - PubMed
-
- White, H. D., and E. W. Taylor. 1976. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 15:5818–5826. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

