Increased air temperature during simulated autumn conditions does not increase photosynthetic carbon gain but affects the dissipation of excess energy in seedlings of the evergreen conifer Jack pine
- PMID: 17259287
- PMCID: PMC1820919
- DOI: 10.1104/pp.106.092312
Increased air temperature during simulated autumn conditions does not increase photosynthetic carbon gain but affects the dissipation of excess energy in seedlings of the evergreen conifer Jack pine
Abstract
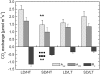
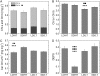
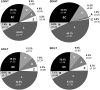
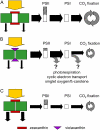
Temperature and daylength act as environmental signals that determine the length of the growing season in boreal evergreen conifers. Climate change might affect the seasonal development of these trees, as they will experience naturally decreasing daylength during autumn, while at the same time warmer air temperature will maintain photosynthesis and respiration. We characterized the down-regulation of photosynthetic gas exchange and the mechanisms involved in the dissipation of energy in Jack pine (Pinus banksiana) in controlled environments during a simulated summer-autumn transition under natural conditions and conditions with altered air temperature and photoperiod. Using a factorial design, we dissected the effects of daylength and temperature. Control plants were grown at either warm summer conditions with 16-h photoperiod and 22 degrees C or conditions representing a cool autumn with 8 h/7 degrees C. To assess the impact of photoperiod and temperature on photosynthesis and energy dissipation, plants were also grown under either cold summer (16-h photoperiod/7 degrees C) or warm autumn conditions (8-h photoperiod/22 degrees C). Photosynthetic gas exchange was affected by both daylength and temperature. Assimilation and respiration rates under warm autumn conditions were only about one-half of the summer values but were similar to values obtained for cold summer and natural autumn treatments. In contrast, photosynthetic efficiency was largely determined by temperature but not by daylength. Plants of different treatments followed different strategies for dissipating excess energy. Whereas in the warm summer treatment safe dissipation of excess energy was facilitated via zeaxanthin, in all other treatments dissipation of excess energy was facilitated predominantly via increased aggregation of the light-harvesting complex of photosystem II. These differences were accompanied by a lower deepoxidation state and larger amounts of beta-carotene in the warm autumn treatment as well as by changes in the abundance of thylakoid membrane proteins compared to the summer condition. We conclude that photoperiod control of dormancy in Jack pine appears to negate any potential for an increased carbon gain associated with higher temperatures during the autumn season.
Figures



Similar articles
-
Increased air temperature during simulated autumn conditions impairs photosynthetic electron transport between photosystem II and photosystem I.Plant Physiol. 2008 May;147(1):402-14. doi: 10.1104/pp.108.117598. Epub 2008 Mar 28. Plant Physiol. 2008. PMID: 18375598 Free PMC article.
-
Photoperiod and temperature constraints on the relationship between the photochemical reflectance index and the light use efficiency of photosynthesis in Pinus strobus.Tree Physiol. 2016 Mar;36(3):311-24. doi: 10.1093/treephys/tpv143. Epub 2016 Feb 3. Tree Physiol. 2016. PMID: 26846980 Free PMC article.
-
Elevated Temperature and CO2 Stimulate Late-Season Photosynthesis But Impair Cold Hardening in Pine.Plant Physiol. 2016 Oct;172(2):802-818. doi: 10.1104/pp.16.00753. Epub 2016 Sep 2. Plant Physiol. 2016. PMID: 27591187 Free PMC article.
-
Champions of winter survival: cold acclimation and molecular regulation of cold hardiness in evergreen conifers.New Phytol. 2021 Jan;229(2):675-691. doi: 10.1111/nph.16904. Epub 2020 Sep 27. New Phytol. 2021. PMID: 32869329 Review.
-
Exploring the metabolic daylength measurement system: implications for photoperiodic growth.New Phytol. 2025 Jan;245(2):503-509. doi: 10.1111/nph.20275. Epub 2024 Nov 15. New Phytol. 2025. PMID: 39544075 Review.
Cited by
-
Increased air temperature during simulated autumn conditions impairs photosynthetic electron transport between photosystem II and photosystem I.Plant Physiol. 2008 May;147(1):402-14. doi: 10.1104/pp.108.117598. Epub 2008 Mar 28. Plant Physiol. 2008. PMID: 18375598 Free PMC article.
-
Effects of warming on chlorophyll degradation and carbohydrate accumulation of Alpine herbaceous species during plant senescence on the Tibetan Plateau.PLoS One. 2014 Sep 18;9(9):e107874. doi: 10.1371/journal.pone.0107874. eCollection 2014. PLoS One. 2014. PMID: 25232872 Free PMC article.
-
Photoperiod and temperature constraints on the relationship between the photochemical reflectance index and the light use efficiency of photosynthesis in Pinus strobus.Tree Physiol. 2016 Mar;36(3):311-24. doi: 10.1093/treephys/tpv143. Epub 2016 Feb 3. Tree Physiol. 2016. PMID: 26846980 Free PMC article.
-
Coordinated regulation of photosynthetic and respiratory components is necessary to maintain chloroplast energy balance in varied growth conditions.J Exp Bot. 2017 Jan 1;68(3):657-671. doi: 10.1093/jxb/erw469. J Exp Bot. 2017. PMID: 28011719 Free PMC article.
-
Sensitivity of cold acclimation to elevated autumn temperature in field-grown Pinus strobus seedlings.Front Plant Sci. 2015 Mar 24;6:165. doi: 10.3389/fpls.2015.00165. eCollection 2015. Front Plant Sci. 2015. PMID: 25852717 Free PMC article.
References
-
- Adams WW, Zarter CR, Ebbert V, Demmig-Adams B (2004) Photoprotective strategies of overwintering evergreens. Bioscience 54 41–49
-
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50 601–639 - PubMed
-
- Bigras FJ, Ryyppö A, Lindström A, Stattin E (2001) Cold acclimation and deacclimation of shoots and roots of conifer seedlings. In FJ Bigras, SJ Colombo, eds, Conifer Cold Hardiness. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 57–88
-
- Bukhov NG, Heber U, Wiese C, Shuvalov VA (2001) Energy dissipation in photosynthesis: does the quenching of chlorophyll fluorescence originate from antenna complexes of photosystem II or from the reaction center? Planta 212 749–758 - PubMed
-
- Cantrell A, McGarvey DJ, Truscott TG, Rancan F, Bohm F (2003) Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch Biochem Biophys 412 47–54 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

