Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages
- PMID: 17259370
- PMCID: PMC2819351
- DOI: 10.2337/db06-0267
Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages
Abstract
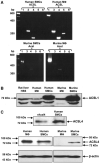
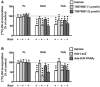
Rosiglitazone is an insulin-sensitizing agent that has recently been shown to exert beneficial effects on atherosclerosis. In addition to peroxisome proliferator-activated receptor (PPAR)-gamma, rosiglitazone can affect other targets, such as directly inhibiting recombinant long-chain acyl-CoA synthetase (ACSL)-4 activity. Because it is unknown if ACSL4 is expressed in vascular cells involved in atherosclerosis, we investigated the ability of rosiglitazone to inhibit ACSL activity and fatty acid partitioning in human and murine arterial smooth muscle cells (SMCs) and macrophages. Human and murine SMCs and human macrophages expressed Acsl4, and rosiglitazone inhibited Acsl activity in these cells. Furthermore, rosiglitazone acutely inhibited partitioning of fatty acids into phospholipids in human SMCs and inhibited fatty acid partitioning into diacylglycerol and triacylglycerol in human SMCs and macrophages through a PPAR-gamma-independent mechanism. Conversely, murine macrophages did not express ACSL4, and rosiglitazone did not inhibit ACSL activity in these cells, nor did it affect acute fatty acid partitioning into cellular lipids. Thus, rosiglitazone inhibits ACSL activity and fatty acid partitioning in human and murine SMCs and in human macrophages through a PPAR-gamma-independent mechanism likely to be mediated by ACSL4 inhibition. Therefore, rosiglitazone might alter the biological effects of fatty acids in these cells and in atherosclerosis.
Figures




Similar articles
-
Rosiglitazone inhibits angiotensin II-induced CTGF expression in vascular smooth muscle cells - role of PPAR-gamma in vascular fibrosis.Biochem Pharmacol. 2007 Jan 15;73(2):185-97. doi: 10.1016/j.bcp.2006.09.019. Epub 2006 Sep 23. Biochem Pharmacol. 2007. PMID: 17074304
-
Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells.J Biol Chem. 2006 Jan 13;281(2):945-50. doi: 10.1074/jbc.M507646200. Epub 2005 Nov 1. J Biol Chem. 2006. PMID: 16263710
-
Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin.Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2480-97. doi: 10.1152/ajpheart.01344.2005. Epub 2006 Jan 20. Am J Physiol Heart Circ Physiol. 2006. PMID: 16428347
-
Role of long-chain acyl-CoA synthetase 4 in formation of polyunsaturated lipid species in hepatic stellate cells.Biochim Biophys Acta. 2015 Feb;1851(2):220-30. doi: 10.1016/j.bbalip.2014.12.003. Epub 2014 Dec 9. Biochim Biophys Acta. 2015. PMID: 25500141
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
Cited by
-
CRISPR/Cas9 Screens Reveal that Hexokinase 2 Enhances Cancer Stemness and Tumorigenicity by Activating the ACSL4-Fatty Acid β-Oxidation Pathway.Adv Sci (Weinh). 2022 Jul;9(21):e2105126. doi: 10.1002/advs.202105126. Epub 2022 May 23. Adv Sci (Weinh). 2022. PMID: 35603967 Free PMC article.
-
Liver-specific knockdown of long-chain acyl-CoA synthetase 4 reveals its key role in VLDL-TG metabolism and phospholipid synthesis in mice fed a high-fat diet.Am J Physiol Endocrinol Metab. 2019 May 1;316(5):E880-E894. doi: 10.1152/ajpendo.00503.2018. Epub 2019 Feb 5. Am J Physiol Endocrinol Metab. 2019. PMID: 30721098 Free PMC article.
-
Targeting ferroptosis opens new avenues for the development of novel therapeutics.Signal Transduct Target Ther. 2023 Sep 21;8(1):372. doi: 10.1038/s41392-023-01606-1. Signal Transduct Target Ther. 2023. PMID: 37735472 Free PMC article. Review.
-
Chlamydia trachomatis growth and development requires the activity of host Long-chain Acyl-CoA Synthetases (ACSLs).Sci Rep. 2016 Mar 18;6:23148. doi: 10.1038/srep23148. Sci Rep. 2016. PMID: 26988341 Free PMC article.
-
ACSL4 Directs Intramuscular Adipogenesis and Fatty Acid Composition in Pigs.Animals (Basel). 2022 Jan 4;12(1):119. doi: 10.3390/ani12010119. Animals (Basel). 2022. PMID: 35011225 Free PMC article.
References
-
- Kornberg A, Pricer WE., Jr Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953;204:329–343. - PubMed
-
- Coleman RA, Van Horn CG, Gonzalez-Baró MR, Lewin TM. Long-chain acyl CoA synthetase isoforms and their functions. In: Haldar D, Das SK, editors. Lipids: Glycerolipid Metabolizing Enzymes. Research Signpost; Trivandrum, India: 2002. pp. 1–15.
-
- Mashek DG, Bornfeldt KE, Coleman RA, Berger J, Bernlohr DA, Black P, DiRusso CC, Farber SA, Guo W, Hashimoto N, Khodiyar V, Kuypers FA, Maltais LJ, Nebert DW, Renieri A, Schaffer JE, Stahl A, Watkins PA, Vasiliou V, Yamamoto TT. Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family. J Lipid Res. 2004;45:1958–1961. - PubMed
-
- Grinberg A, Park KW. Nuclear peroxisome proliferator-activated receptors and thiazolidinediones. Int Anesthesiol Clin. 2005;43:1–21. - PubMed
-
- Chiquette E, Ramirez G, DeFronzo R. A meta-analysis comparing the effects of thiazolidinediones on cardiovascular risk factors. Arch Intern Med. 2004;164:2097–2104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

