IGF-binding protein-2 protects against the development of obesity and insulin resistance
- PMID: 17259371
- PMCID: PMC4295171
- DOI: 10.2337/db06-0436
IGF-binding protein-2 protects against the development of obesity and insulin resistance
Abstract
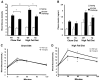
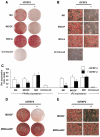
Proliferation of adipocyte precursors and their differentiation into mature adipocytes contributes to the development of obesity in mammals. IGF-I is a potent mitogen and important stimulus for adipocyte differentiation. The biological actions of IGFs are closely regulated by a family of IGF-binding proteins (IGFBPs), which exert predominantly inhibitory effects. IGFBP-2 is the principal binding protein secreted by differentiating white preadipocytes, suggesting a potential role in the development of obesity. We have generated transgenic mice overexpressing human IGFBP-2 under the control of its native promoter, and we show that overexpression of IGFBP-2 is associated with reduced susceptibility to obesity and improved insulin sensitivity. Whereas wild-type littermates developed glucose intolerance and increased blood pressure with aging, mice overexpressing IGFBP-2 were protected. Furthermore, when fed a high-fat/high-energy diet, IGFBP-2-overexpressing mice were resistant to the development of obesity and insulin resistance. This lean phenotype was associated with decreased leptin levels, increased glucose sensitivity, and lower blood pressure compared with wild-type animals consuming similar amounts of high-fat diet. Our in vitro data suggest a direct effect of IGFBP-2 preventing adipogenesis as indicated by the ability of recombinant IGFBP-2 to impair 3T3-L1 differentiation. These findings suggest an important, novel role for IGFBP-2 in obesity prevention.
Figures
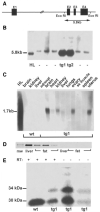
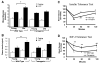
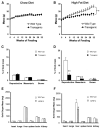
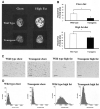
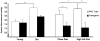
References
-
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. - PubMed
-
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. - PubMed
-
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. - PubMed
-
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. - PubMed
-
- Schmidt W, Poll-Jordan G, Loffler G. Adipose conversion of 3T3-L1 cells in a serum-free culture system depends on epidermal growth factor, insulin-like growth factor I, corticosterone, and cyclic AMP. J Biol Chem. 1990;265:15489–15495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

