Resolving the Two "Bony" Faces of PPAR-gamma
- PMID: 17259664
- PMCID: PMC1679961
- DOI: 10.1155/PPAR/2006/27489
Resolving the Two "Bony" Faces of PPAR-gamma
Abstract
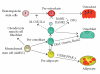
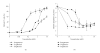
Bone loss with aging results from attenuated and unbalanced bone turnover that has been associated with a decreased number of bone forming osteoblasts, an increased number of bone resorbing osteoclasts, and an increased number of adipocytes (fat cells) in the bone marrow. Osteoblasts and adipocytes are derived from marrow mesenchymal stroma/stem cells (MSC). The milieu of intracellular and extracellular signals that controls MSC lineage allocation is diverse. The adipocyte-specific transcription factor peroxisome proliferator-activated receptor-gamma (PPAR-gamma) acts as a critical positive regulator of marrow adipocyte formation and as a negative regulator of osteoblast development. In vivo, increased PPAR-gamma activity leads to bone loss, similar to the bone loss observed with aging, whereas decreased PPAR-gamma activity results in increased bone mass. Emerging evidence suggests that the pro-adipocytic and the anti-osteoblastic properties of PPAR-gamma are ligand-selective, suggesting the existence of multiple mechanisms by which PPAR-gamma controls bone mass and fat mass in bone.
Figures
Similar articles
-
Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat.Bone. 2006 Jan;38(1):74-84. doi: 10.1016/j.bone.2005.07.008. Epub 2005 Aug 30. Bone. 2006. PMID: 16137931 Free PMC article.
-
Molecular Mechanisms of PPAR-γ Governing MSC Osteogenic and Adipogenic Differentiation.Curr Stem Cell Res Ther. 2016;11(3):255-64. doi: 10.2174/1574888x10666150531173309. Curr Stem Cell Res Ther. 2016. PMID: 26027680 Review.
-
β-catenin directly sequesters adipocytic and insulin sensitizing activities but not osteoblastic activity of PPARγ2 in marrow mesenchymal stem cells.PLoS One. 2012;7(12):e51746. doi: 10.1371/journal.pone.0051746. Epub 2012 Dec 18. PLoS One. 2012. PMID: 23272157 Free PMC article.
-
Partial agonist, telmisartan, maintains PPARγ serine 112 phosphorylation, and does not affect osteoblast differentiation and bone mass.PLoS One. 2014 May 8;9(5):e96323. doi: 10.1371/journal.pone.0096323. eCollection 2014. PLoS One. 2014. PMID: 24810249 Free PMC article.
-
At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives.Int J Mol Sci. 2020 Mar 26;21(7):2277. doi: 10.3390/ijms21072277. Int J Mol Sci. 2020. PMID: 32224846 Free PMC article. Review.
Cited by
-
14-3-3γ Regulates Lipopolysaccharide-Induced Inflammatory Responses and Lactation in Dairy Cow Mammary Epithelial Cells by Inhibiting NF-κB and MAPKs and Up-Regulating mTOR Signaling.Int J Mol Sci. 2015 Jul 22;16(7):16622-41. doi: 10.3390/ijms160716622. Int J Mol Sci. 2015. PMID: 26204835 Free PMC article.
-
Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation.Nutrients. 2020 Apr 2;12(4):989. doi: 10.3390/nu12040989. Nutrients. 2020. PMID: 32252359 Free PMC article. Review.
-
Marrow fat metabolism is linked to the systemic energy metabolism.Bone. 2012 Feb;50(2):534-9. doi: 10.1016/j.bone.2011.06.032. Epub 2011 Jul 4. Bone. 2012. PMID: 21757043 Free PMC article. Review.
-
Plasma phosphatidylcholine concentrations of polyunsaturated fatty acids are differentially associated with hip bone mineral density and hip fracture in older adults: the Framingham Osteoporosis Study.J Bone Miner Res. 2012 May;27(5):1222-30. doi: 10.1002/jbmr.1581. J Bone Miner Res. 2012. PMID: 22392875 Free PMC article.
-
Effect of retinoic acid and vitamin D3 on osteoblast differentiation and activity in aging.J Bone Miner Metab. 2016 Jan;34(1):65-78. doi: 10.1007/s00774-014-0642-2. Epub 2015 Feb 18. J Bone Miner Metab. 2016. PMID: 25691285
References
-
- Robey PG, Bianco P. Cellular mechanisms of age-related bone loss. In: Rosen CGJ, Bilezikian JP, editors. The Aging Skeleton. San Diego, Califf: Academic Press; 1999. pp. 145–157.
-
- Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19(suppl 5):421–428. - PubMed
-
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. Journal of Cellular Biochemistry. 2006;98(2):251–266. - PubMed
-
- Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-γ2ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143(6):2376–2384. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

