The tripartite motif of nuclear factor 7 is required for its association with transcriptional units
- PMID: 17261593
- PMCID: PMC1899906
- DOI: 10.1128/MCB.01968-06
The tripartite motif of nuclear factor 7 is required for its association with transcriptional units
Abstract
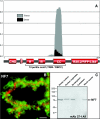
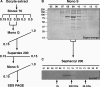
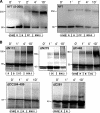
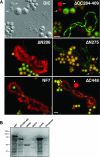
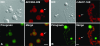
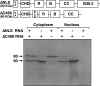
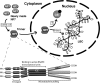
In amphibian oocytes, the maternal nuclear factor NF7 associates with the elongating pre-mRNAs present on the numerous lateral loops of the lampbrush chromosomes. Here, we have purified NF7 from an oocyte extract by using a combination of ion-exchange chromatography and gel filtration chromatography and demonstrated for the first time that nucleoplasmic NF7 exists primarily as free homotrimers. We confirmed the in vivo homotrimerization of NF7 by using a glutaraldehyde cross-linking assay, and we further showed that it only requires the coiled-coil domain of the NF7 tripartite motif/RBCC motif. Interestingly, we also obtained evidence that NF7 is recruited to the nucleus as a homotrimer, and expression of several mutated forms of NF7 in oocytes demonstrated that both the coiled coil and B box of NF7 are required for its chromosomal association. Together, these data strongly suggest that the interaction of NF7 with the active transcriptional units of RNA polymerase II is mediated by a trimeric B box. Finally, and in agreement with a role for NF7 in pre-mRNA maturation, we obtained evidence supporting the idea that NF7 associates with Cajal bodies.
Figures
Similar articles
-
RNA polymerase III in Cajal bodies and lampbrush chromosomes of the Xenopus oocyte nucleus.Mol Biol Cell. 2002 Oct;13(10):3466-76. doi: 10.1091/mbc.e02-05-0281. Mol Biol Cell. 2002. PMID: 12388750 Free PMC article.
-
Lampbrush chromosomes and associated bodies: new insights into principles of nuclear structure and function.Chromosome Res. 2002;10(3):177-200. doi: 10.1023/a:1015227020652. Chromosome Res. 2002. PMID: 12067208 Review.
-
Subnuclear localization and Cajal body targeting of transcription elongation factor TFIIS in amphibian oocytes.Mol Biol Cell. 2003 Mar;14(3):1255-67. doi: 10.1091/mbc.e02-09-0601. Mol Biol Cell. 2003. PMID: 12631738 Free PMC article.
-
Cajal bodies and histone locus bodies in Drosophila and Xenopus.Cold Spring Harb Symp Quant Biol. 2010;75:313-20. doi: 10.1101/sqb.2010.75.005. Epub 2010 Nov 3. Cold Spring Harb Symp Quant Biol. 2010. PMID: 21047905
-
Use of intact Xenopus oocytes in nucleocytoplasmic transport studies.Methods Mol Biol. 2006;322:301-14. doi: 10.1007/978-1-59745-000-3_21. Methods Mol Biol. 2006. PMID: 16739732 Review.
Cited by
-
Association of modified cytosines and the methylated DNA-binding protein MeCP2 with distinctive structural domains of lampbrush chromatin.Chromosome Res. 2012 Dec;20(8):925-42. doi: 10.1007/s10577-012-9324-x. Chromosome Res. 2012. PMID: 23149574 Free PMC article.
-
Lampbrush chromosomes enable study of cohesin dynamics.Chromosome Res. 2009;17(2):165-84. doi: 10.1007/s10577-008-9015-9. Chromosome Res. 2009. PMID: 19308699
-
Novel domains in the hnRNP G/RBMX protein with distinct roles in RNA binding and targeting nascent transcripts.Nucleus. 2010 Jan-Feb;1(1):109-22. doi: 10.4161/nucl.1.1.10857. Nucleus. 2010. PMID: 21327109 Free PMC article.
-
The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association.J Virol. 2008 Dec;82(23):11495-502. doi: 10.1128/JVI.01548-08. Epub 2008 Sep 17. J Virol. 2008. PMID: 18799578 Free PMC article.
-
The RanGTP Pathway: From Nucleo-Cytoplasmic Transport to Spindle Assembly and Beyond.Front Cell Dev Biol. 2016 Jan 11;3:82. doi: 10.3389/fcell.2015.00082. eCollection 2015. Front Cell Dev Biol. 2016. PMID: 26793706 Free PMC article. Review.
References
-
- Abbadie, C., D. Boucher, J. Charlemagne, and J.-C. Lacroix. 1987. Immunolocalization of three oocyte nuclear proteins during oogenesis and embryogenesis in Pleurodeles. Development 101:715-728.
-
- Barlow, P. N., B. Luisi, A. Milner, M. Elliott, and R. Everett. 1994. Structure of the C3HC4 domain by 1H-nuclear magnetic resonance spectroscopy. A new structural class of zinc-finger. J. Mol. Biol. 237:201-211. - PubMed
-
- Beenders, B., E. Watrin, V. Legagneux, I. Kireev, and M. Bellini. 2003. Distribution of XCAP-E and XCAP-D2 in the Xenopus oocyte nucleus. Chromosome Res. 11:549-564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
