Dss1 interaction with Brh2 as a regulatory mechanism for recombinational repair
- PMID: 17261595
- PMCID: PMC1899899
- DOI: 10.1128/MCB.01907-06
Dss1 interaction with Brh2 as a regulatory mechanism for recombinational repair
Abstract
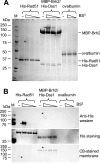
Brh2, the BRCA2 ortholog in Ustilago maydis, enables recombinational repair of DNA by controlling Rad51 and is in turn regulated by Dss1. Interplay with Rad51 is conducted via the BRC element located in the N-terminal region of the protein and through an unrelated domain, CRE, at the C terminus. Mutation in either BRC or CRE severely reduces functional activity, but repair deficiency of the brh2 mutant can be complemented by expressing BRC and CRE on different molecules. This intermolecular complementation is dependent upon the presence of Dss1. Brh2 molecules associate through the region overlapping with the Dss1-interacting domain to form at least dimer-sized complexes, which in turn, can be dissociated by Dss1 to monomer. We propose that cooperation between BRC and CRE domains and the Dss1-provoked dissociation of Brh2 complexes are requisite features of Brh2's molecular mechanism.
Figures








Similar articles
-
Brh2-Dss1 interplay enables properly controlled recombination in Ustilago maydis.Mol Cell Biol. 2005 Apr;25(7):2547-57. doi: 10.1128/MCB.25.7.2547-2557.2005. Mol Cell Biol. 2005. PMID: 15767662 Free PMC article.
-
Dss1 Regulates Association of Brh2 with Rad51.Biochemistry. 2017 Jul 5;56(26):3318-3327. doi: 10.1021/acs.biochem.7b00184. Epub 2017 Jun 26. Biochemistry. 2017. PMID: 28616972 Free PMC article.
-
Dss1 regulates interaction of Brh2 with DNA.Biochemistry. 2009 Dec 22;48(50):11929-38. doi: 10.1021/bi901775j. Biochemistry. 2009. PMID: 19919104 Free PMC article.
-
The BRCA2-interacting protein DSS1 is vital for DNA repair, recombination, and genome stability in Ustilago maydis.Mol Cell. 2003 Oct;12(4):1043-9. doi: 10.1016/s1097-2765(03)00367-8. Mol Cell. 2003. PMID: 14580353
-
The homologous recombination system of Ustilago maydis.Fungal Genet Biol. 2008 Aug;45 Suppl 1(Suppl 1):S31-9. doi: 10.1016/j.fgb.2008.04.006. Epub 2008 May 23. Fungal Genet Biol. 2008. PMID: 18502156 Free PMC article. Review.
Cited by
-
DSSylation, a novel protein modification targets proteins induced by oxidative stress, and facilitates their degradation in cells.Protein Cell. 2014 Feb;5(2):124-40. doi: 10.1007/s13238-013-0018-8. Epub 2014 Feb 11. Protein Cell. 2014. PMID: 24515614 Free PMC article.
-
Molding BRCA2 function through its interacting partners.Cell Cycle. 2015;14(21):3389-95. doi: 10.1080/15384101.2015.1093702. Cell Cycle. 2015. PMID: 26566862 Free PMC article. Review.
-
Structure and mechanism of action of the BRCA2 breast cancer tumor suppressor.Nat Struct Mol Biol. 2014 Nov;21(11):962-968. doi: 10.1038/nsmb.2899. Epub 2014 Oct 5. Nat Struct Mol Biol. 2014. PMID: 25282148 Free PMC article.
-
Cancer-causing BRCA2 missense mutations disrupt an intracellular protein assembly mechanism to disable genome maintenance.Nucleic Acids Res. 2021 Jun 4;49(10):5588-5604. doi: 10.1093/nar/gkab308. Nucleic Acids Res. 2021. PMID: 33978741 Free PMC article.
-
The fungus Ustilago maydis and humans share disease-related proteins that are not found in Saccharomyces cerevisiae.BMC Genomics. 2007 Dec 20;8:473. doi: 10.1186/1471-2164-8-473. BMC Genomics. 2007. PMID: 18096044 Free PMC article.
References
-
- Baillat, D., M. A. Hakimi, A. M. Naar, A. Shilatifard, N. Cooch, and R. Shiekhattar. 2005. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123:631-640. - PubMed
-
- Chen, C. F., P. L. Chen, Q. Zhong, Z. D. Sharp, and W. H. Lee. 1999. Expression of BRC repeats in breast cancer cells disrupts the BRCA2-Rad51 complex and leads to radiation hypersensitivity and loss of G(2)/M checkpoint control. J. Biol. Chem. 274:32931-32935. - PubMed
-
- Clontech Laboratories. 2001. Yeast protocols handbook. PT3024-1. Clontech Laboratories, Palo Alto, CA.
-
- Davies, A. A., J. Y. Masson, M. J. McIlwraith, A. Z. Stasiak, A. Stasiak, A. R. Venkitaraman, and S. C. West. 2001. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell 7:273-282. - PubMed
-
- Esashi, F., N. Christ, J. Gannon, Y. Liu, T. Hunt, M. Jasin, and S. C. West. 2005. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434:598-604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
