Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum
- PMID: 17261598
- PMCID: PMC1865674
- DOI: 10.1128/IAI.01473-06
Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum
Abstract
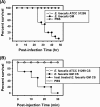
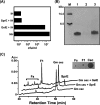
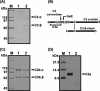
We isolated Enterococcus faecalis from the body fluids of dead larvae of the greater wax moth, Galleria mellonella. Extracellular gelatinase (GelE) and serine protease (SprE), both of which are considered putative virulence factors of E. faecalis, were purified from the culture supernatant of E. faecalis. In an attempt to elucidate their virulence mechanisms, purified GelE and SprE were injected into hemolymph of G. mellonella and evaluated with regard to their effects on the immune system of insect hemolymph. As a result, it was determined that E. faecalis GelE degraded an inducible antimicrobial peptide (Gm cecropin) which is known to perform a critical role in host defense during the early phase of microbial infection. The results obtained from the G. mellonella-E. faecalis infection model compelled us to assess the virulence activity of GelE against the complement system in human serum. E. faecalis GelE hydrolyzed C3a and also mediated the degradation of the alpha chain of C3b, thereby inhibiting opsonization and the formation of the membrane attack complex resultant from the activation of the complement cascade triggered by C3 activation. In contrast, E. faecalis SprE exhibited no virulence effect against the immune system of insect hemolymph or human serum tested in this study.
Figures



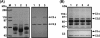
References
-
- Akesson, P., A. G. Sjoholm, and L. Bjorck. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271:1081-1088. - PubMed
-
- Baldassarri, L., R. Creti, L. Montanaro, G. Orefici, and C. R. Arciola. 2005. Pathogenesis of implant infections by enterococci. Int. J. Artif. Organs 28:1101-1109. - PubMed
-
- Coburn, P. S., and M. S. Gilmore. 2003. The Enterococcus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell. Microbiol. 5:661-669. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

