Safety and immunogenicity of an enterotoxigenic Escherichia coli vaccine patch containing heat-labile toxin: use of skin pretreatment to disrupt the stratum corneum
- PMID: 17261601
- PMCID: PMC1865773
- DOI: 10.1128/IAI.01740-06
Safety and immunogenicity of an enterotoxigenic Escherichia coli vaccine patch containing heat-labile toxin: use of skin pretreatment to disrupt the stratum corneum
Abstract
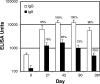
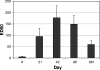
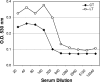
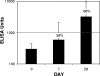
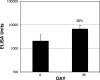
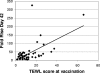
Transcutaneous immunization allows safe delivery of native heat-labile enterotoxin (LT) from Escherichia coli via application of a simple patch. Physical disruption of the stratum corneum can improve the efficiency of delivery. In the current study, the stratum corneum was disrupted using an electrocardiogram prep pad prior to patch application. The effects were quantified using transepidermal water loss (TEWL) and were correlated with the immune responses. Sixty adults received 50 microg of LT from three lots of LT (20 adults per group) administered in a patch on days 0 and 21. The immunizations were well tolerated. There were no differences in the anti-LT immunoglobulin G (IgG) titers between the three LT lots; the seroconversion rate was 100%, and the mean anti-LT IgG titer was 12,185 enzyme-linked immunosorbent assay units (EU) (a 24-fold increase). TEWL measurements obtained at the time of the second immunization were found to correlate with the day 42 individual increases in the anti-LT IgG titer (r = 0.59, P < 0.001). In a comparative assessment of the immune responses, sera after an LT+ ST+ (E2447A) oral ETEC challenge, which induced moderate to severe diarrhea in 81% of the recipients, had anti-LT IgG titers of 3,245 EU (a 10.8-fold increase). Similarly, the anti-LT IgG titer after administration of an oral cholera toxin B subunit-containing cholera vaccine, which cross-reacts with LT and protects against LT and LT/heat-stable toxin ETEC disease in the field, was 6,741 EU (a 3.3-fold increase). This study confirmed that a well-tolerated regimen for stratum corneum disruption before vaccine patch application results in robust immunity comparable to natural immunity and vaccine-induced immunity and that the magnitude of stratum corneum disruption correlates with the immune response.
Figures

References
-
- Black, R. E. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. - PubMed
-
- Clemens, J., S. Savarino, R. Abu-Elyazeed, M. Safwat, M. Rao, T. Wierzba, A. M. Svennerholm, J. Holmgren, R. Frenck, E. Park, and A. Naficy. 2004. Development of pathogenicity-driven definitions of outcomes for a field trial of a killed oral vaccine against enterotoxigenic Escherichia coli in Egypt: application of an evidence-based method. J. Infect. Dis. 189:2299-2307. - PubMed
-
- Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, P. K. Neogy, B. Stanton, N. Huda, M. U. Khan, B. A. Kay, M. R. Khan, et al. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158:372-377. - PubMed
-
- Craig, J. 1965. The effect of cholera stool and culture filtrates on the skin of guinea pigs and rabbits, p. 153-158. Proceedings of the Cholera Research Symposium, January 24-29, 1965, Honolulu, HI. Public Health Service publication no. 1328. U.S. Government Printing Office, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

